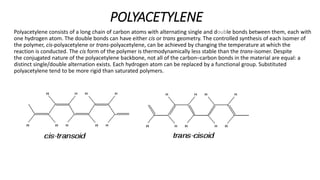
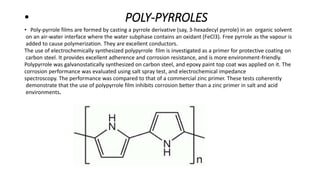
The document explores conducting polymers, particularly focusing on polyacetylene, polyaniline, and polypyrrole, highlighting their conductive properties and applications in various technologies. It outlines the mechanisms of conduction, the importance of doping, and the synthesis processes for these materials. The conclusion emphasizes that conductive polymers serve as semiconductors when undoped and become conductors upon doping.