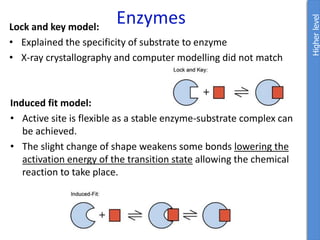
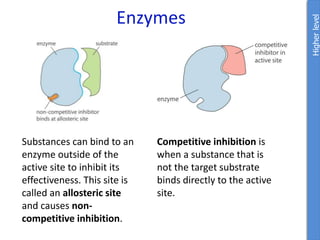
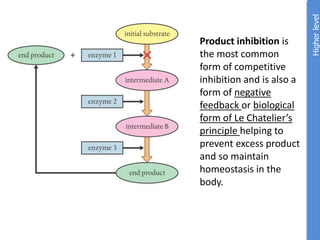
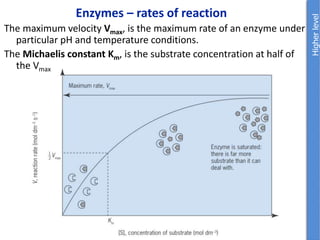
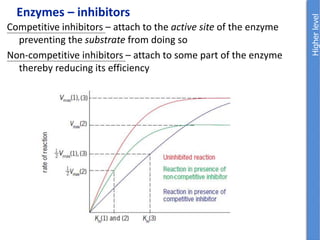
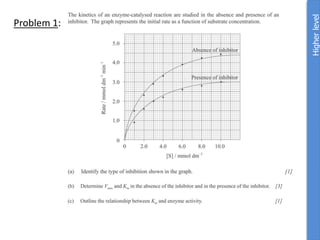
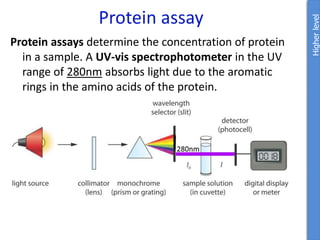
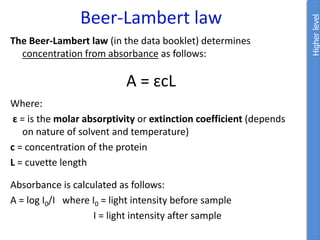
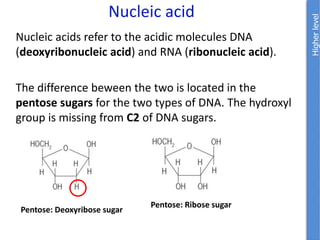
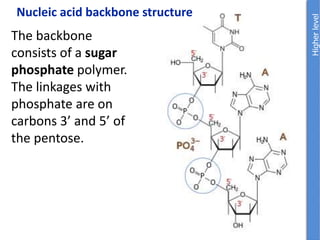
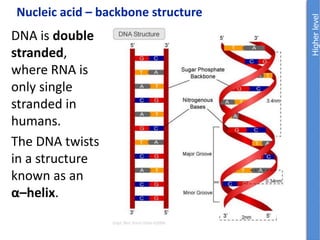
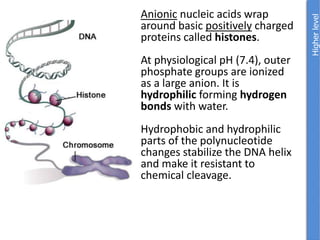
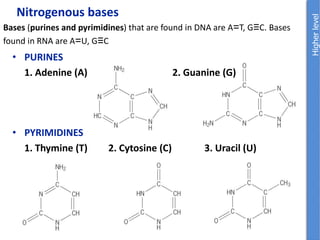
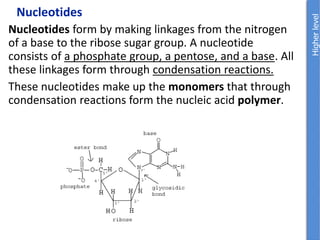
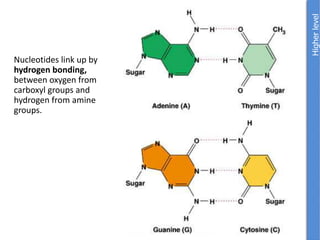
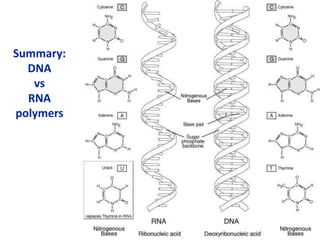
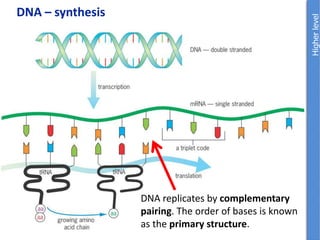
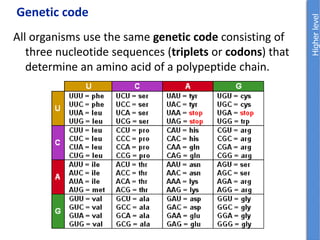
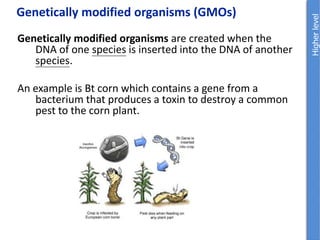
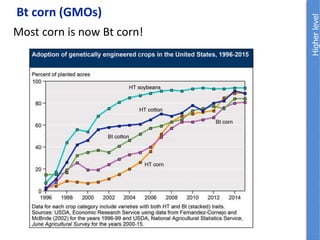
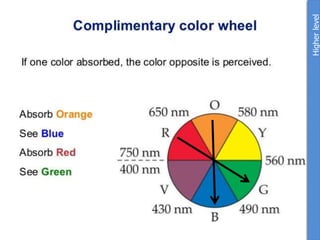
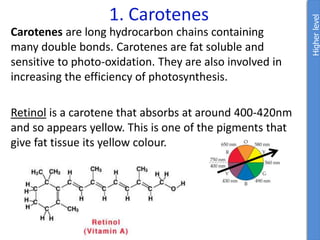
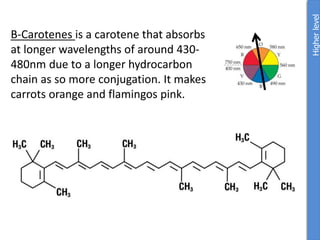
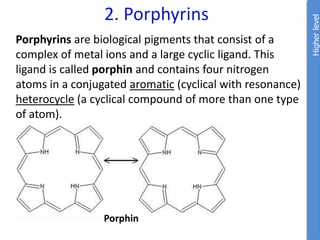
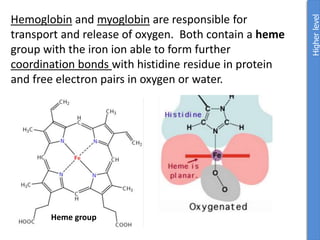
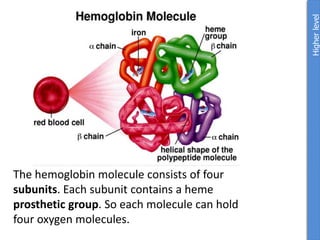
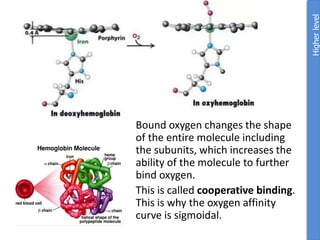
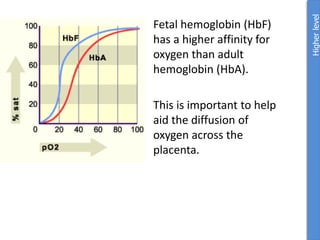
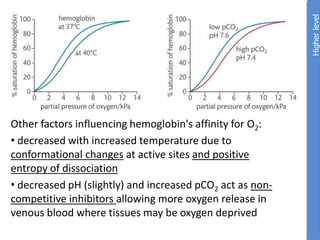
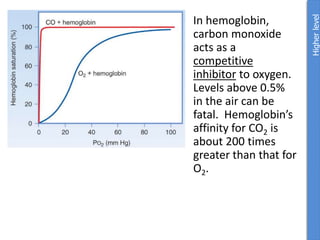
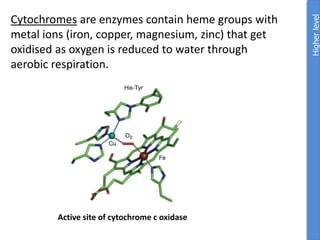
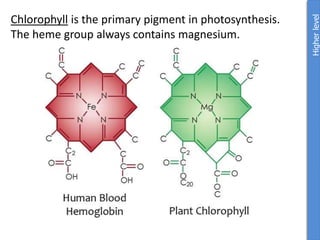
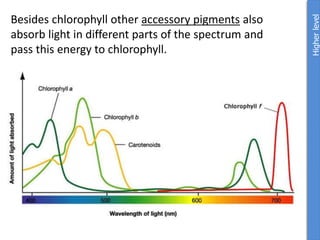
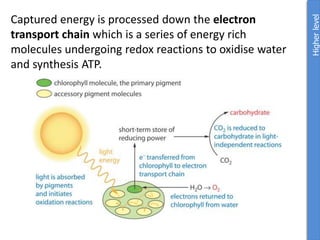
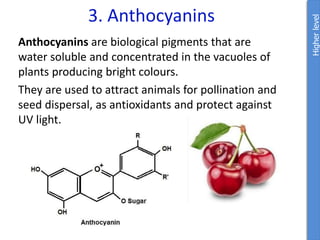
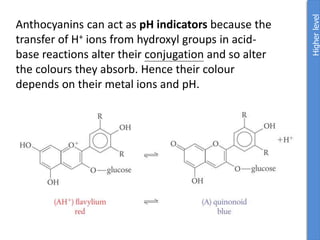
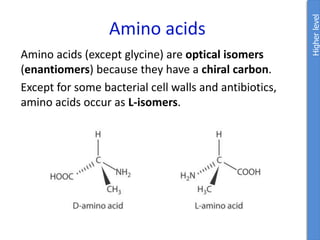
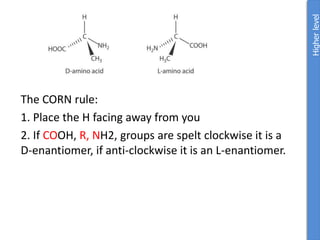
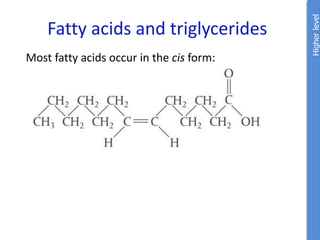
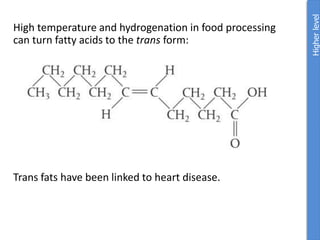
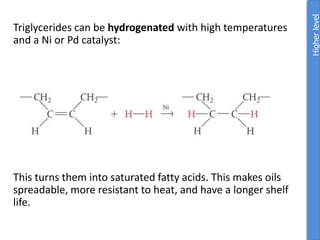
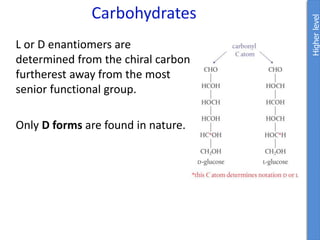
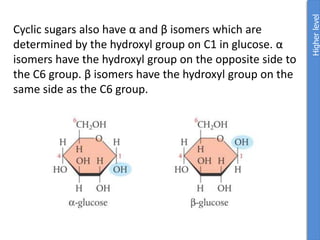
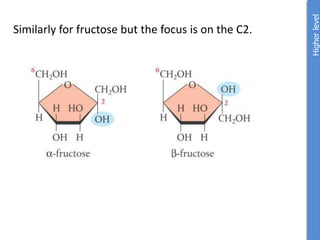
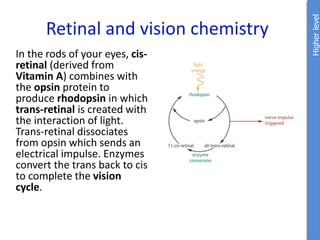
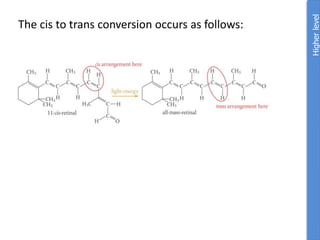
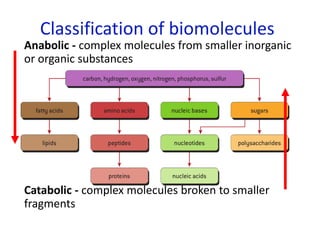
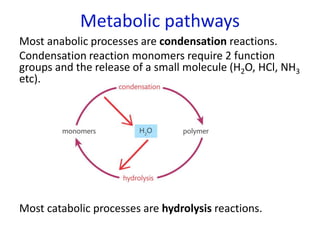
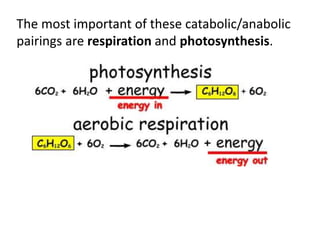
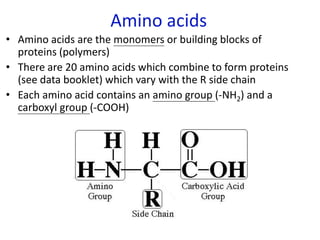
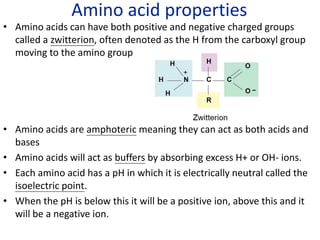
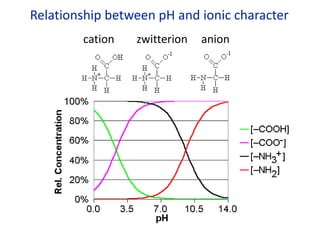
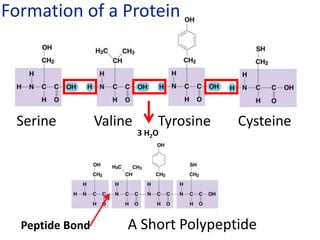
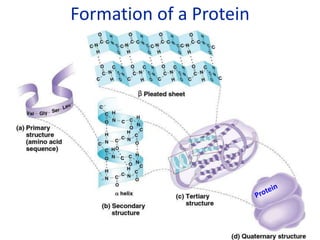
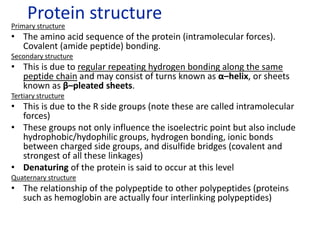
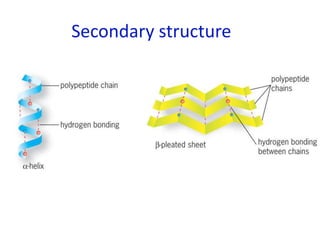
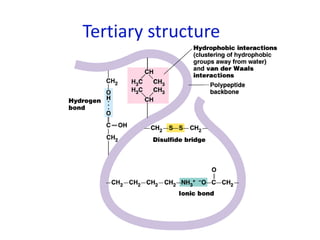
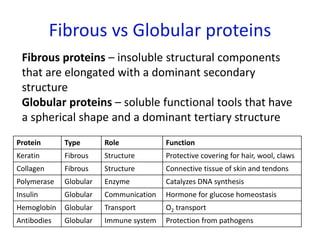
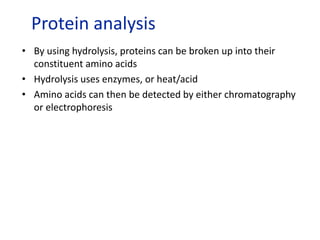
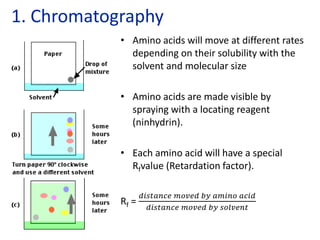
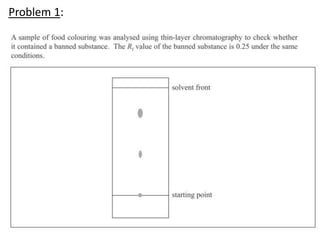
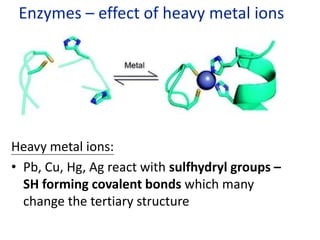
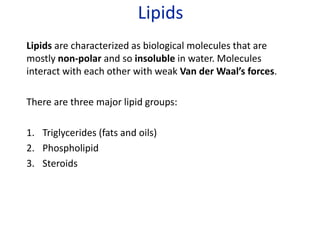
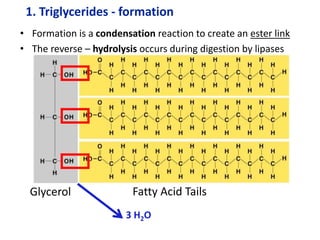
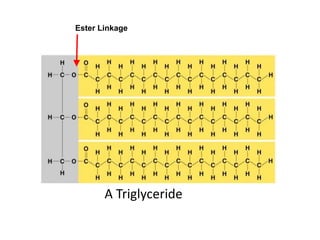
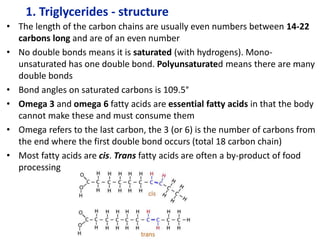
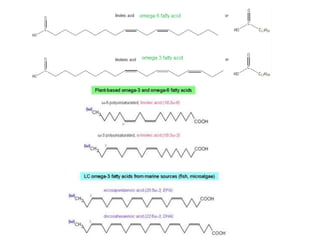
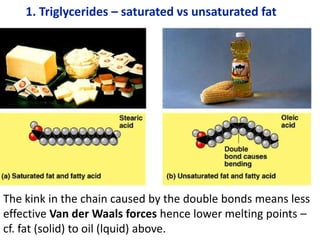
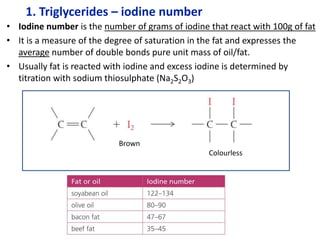
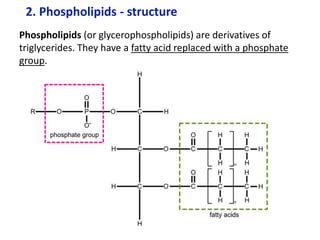
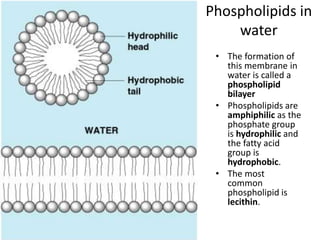
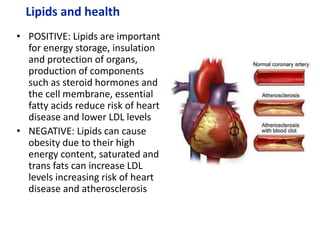
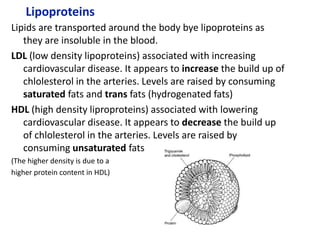
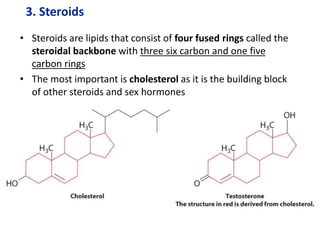
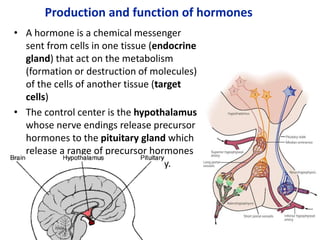
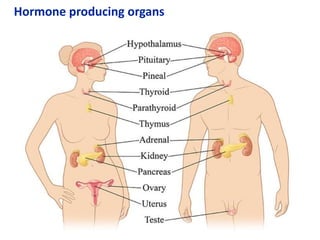
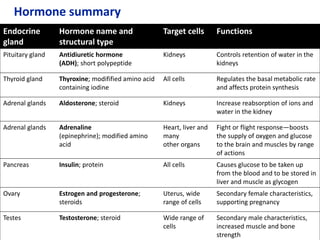
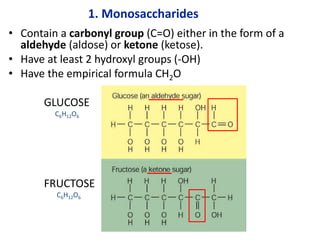
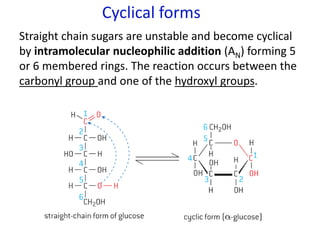
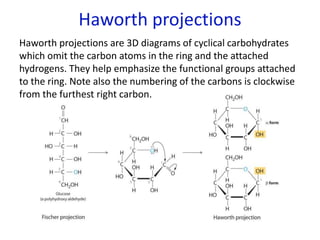
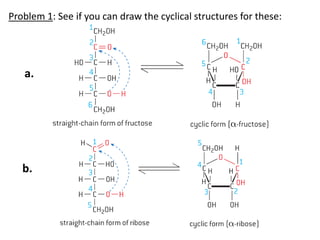
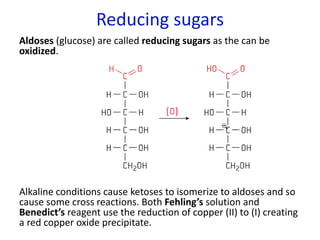
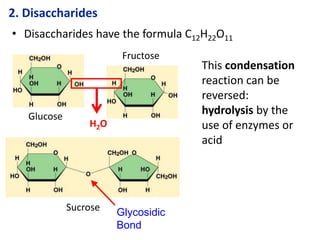
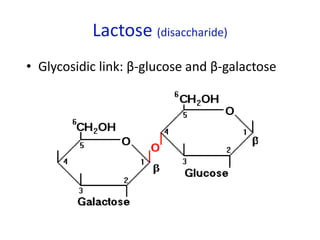
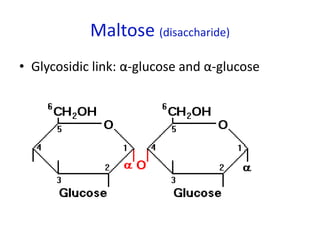
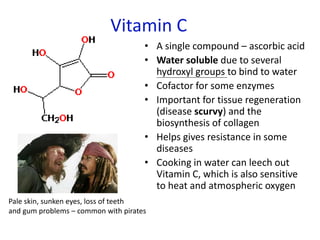
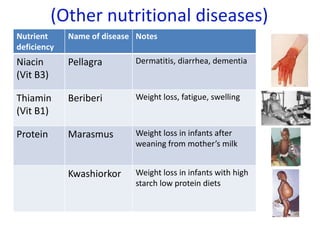
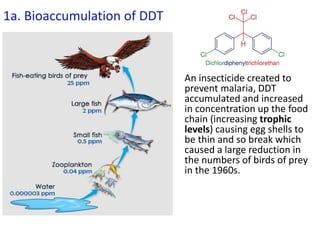
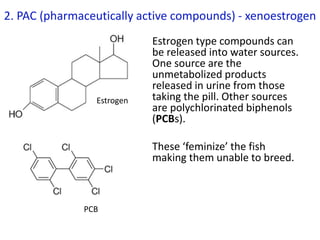
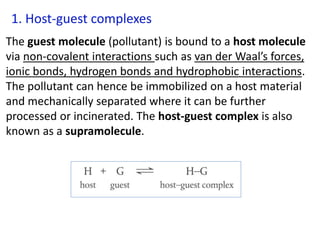
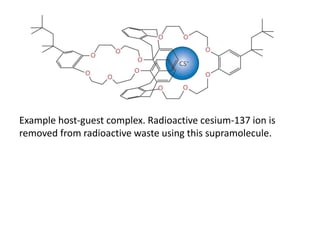
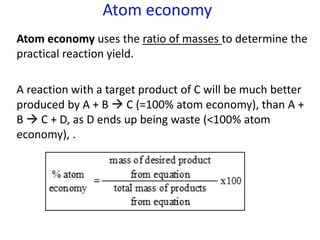
The document provides an overview of biochemistry concepts relevant to higher-level IB Chemistry, focusing on biological molecules, metabolic processes, and their roles in cellular function. It covers topics such as the distinction between catabolism and anabolism, the structure and function of proteins, lipids, enzymes, and carbohydrates. Additionally, it highlights their chemical properties, interactions, and implications for health and metabolism.




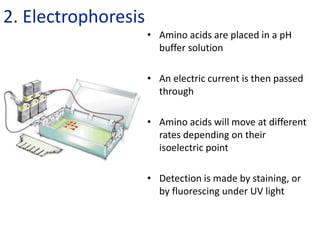

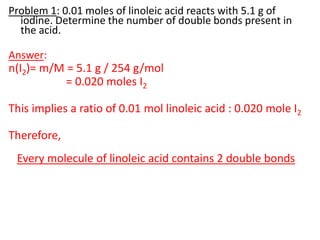



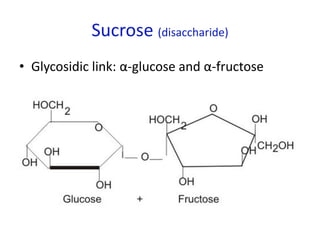











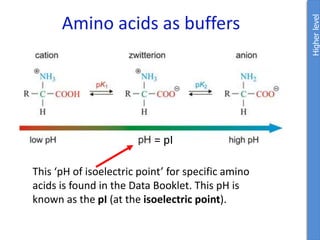
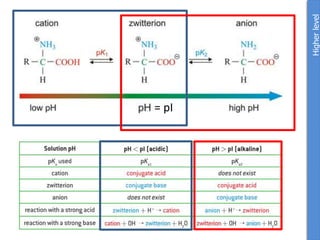
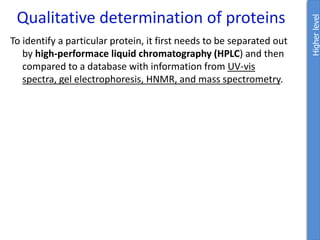
![Problem 1: Calculate the pH of an aqueous solution that contains
0.8M zwitterionic and 0.2M anionic forms of serine.
Solution contains anions therefore high pH due to H+ removed.
pKa = 9.1 zwitterion anion + H+
Therefore zwitterion is the acid and anion is the conj base.
From data booklet:
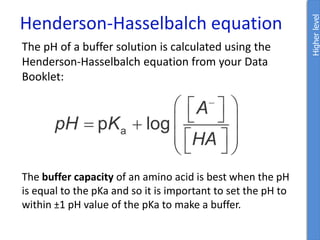
pH = pKa + log ([A-]/[HA])
= pKa + log ([anion]/[zwitterion])
= 9.1 + log (0.2/0.8)
= 9.1 + -0.6
= 8.5
Higher
level](https://image.slidesharecdn.com/ibbiochemistrycompletetopicslandhl-240503124125-c20f57d2/85/Ib-Biochemistry-Complete-Topic-SL-and-HL-ppt-101-320.jpg)
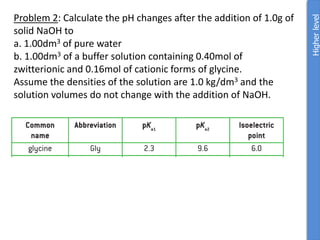
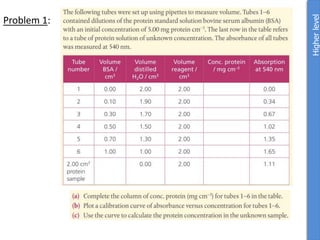
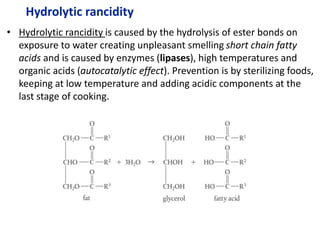
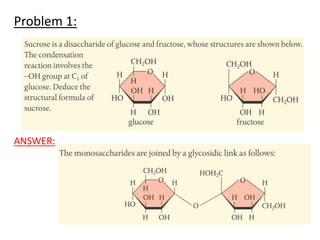
![a. NaOH Na+ + OH-
n(NaOH) = m/M = 1g/40g/mol = 0.025mol NaOH
1 mol NaOH : 1 mol OH-
Therefore [OH] = 0.025mol
pOH = -log [OH] = -log0.025 = 1.059
pKw = pOH + pH
pH = 14 – pOH = 14-1.6 = 12.4
pH water is 7 so change in pH = 12.4 – 7.0 = 5.4
Higher
level](https://image.slidesharecdn.com/ibbiochemistrycompletetopicslandhl-240503124125-c20f57d2/85/Ib-Biochemistry-Complete-Topic-SL-and-HL-ppt-103-320.jpg)
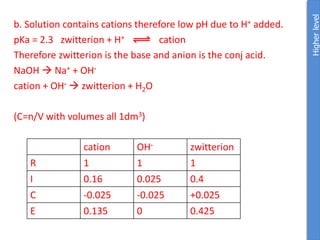
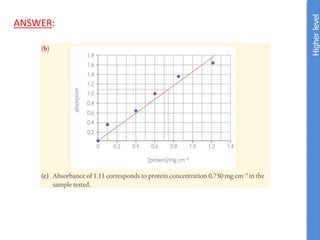
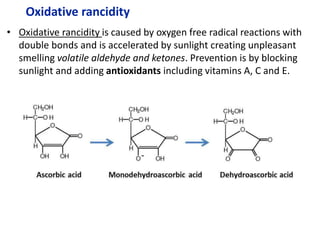
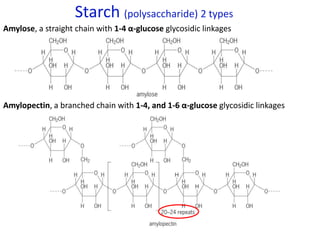
![From data booklet:
pH(before) = pKa + log ([A-]/[HA])
= pKa + log ([zwitterion]/[cation])
= 2.3 + log (0.4/0.16)
= 2.3 + 0.4
= 2.7
pH(after) = pKa + log ([A-]/[HA])
= pKa + log ([zwitterion]/[cation])
= 2.3 + log (0.425/0.135)
= 2.3 + 0.5
= 2.8
Therefore the change in pH = 2.8-2.7 = 0.1
*The comparison of parts a and b show the effectiveness of a
buffer to resist pH changes.
Higher
level](https://image.slidesharecdn.com/ibbiochemistrycompletetopicslandhl-240503124125-c20f57d2/85/Ib-Biochemistry-Complete-Topic-SL-and-HL-ppt-105-320.jpg)
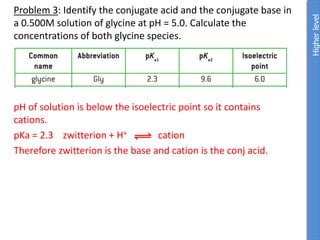
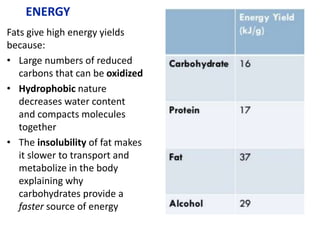
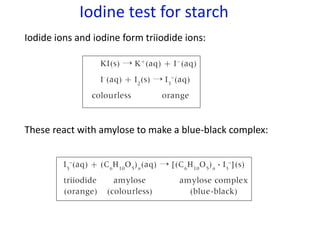
![Higher
level
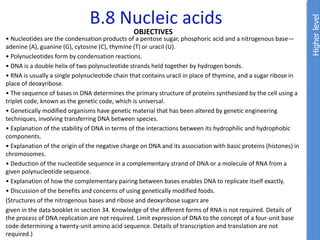
From data booklet:
pH = pKa + log ([A-]/[HA])
5.0 = 2.3 + log ([zwitterion]/[cation])
log ([zwitterion]/[cation]) = 2.7
[zwitterion]/[cation] = 501
If [cation] = x, then [zwitterion] = 501x
Total concentration is x + 501x = 0.500M
502x = 0.500M
x = 0.0001M
(We already know this is not an effective buffer because the pH is more
than 1 pH value away from the pKa)](https://image.slidesharecdn.com/ibbiochemistrycompletetopicslandhl-240503124125-c20f57d2/85/Ib-Biochemistry-Complete-Topic-SL-and-HL-ppt-107-320.jpg)