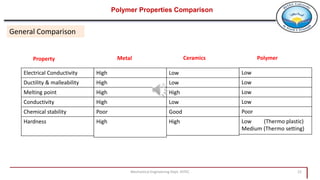
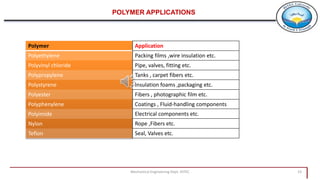
This document provides an overview of polymers including:
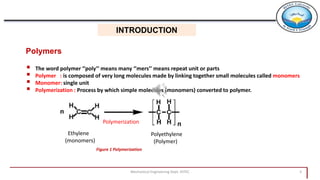
1. Polymers are composed of long molecules made by linking small repeating units called monomers through polymerization. Common examples are polyethylene, nylon, and polyvinyl chloride.
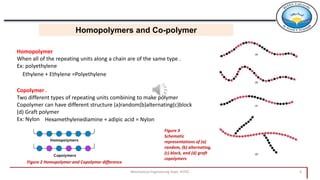
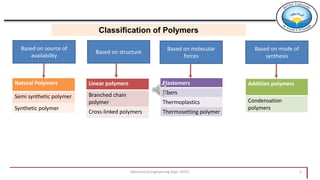
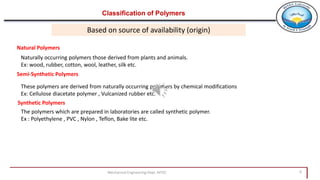
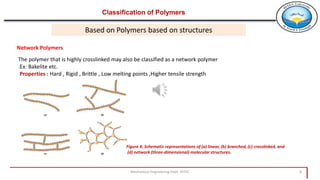
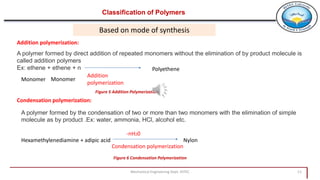
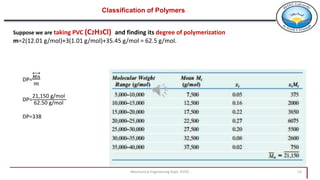
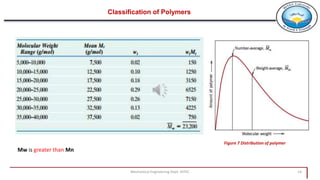
2. Polymers can be classified based on their source, structure, molecular forces, and synthesis method. Some key classifications include natural vs synthetic, linear vs branched structures, thermoplastics vs thermosets, and addition vs condensation polymerization.
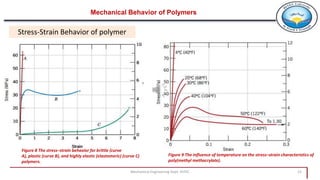
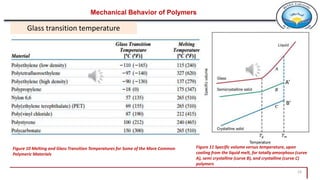
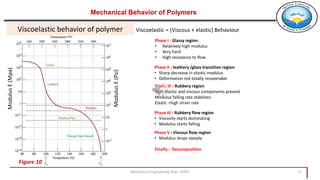
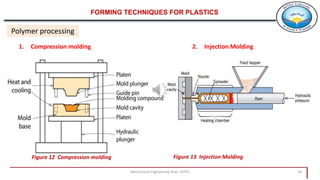
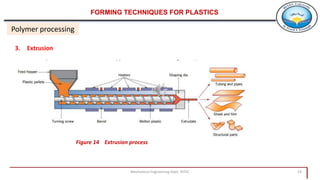
3. Common polymer processing techniques include compression molding, injection molding, and extrusion. Polymers also exhibit viscoelastic behavior and properties that depend on factors like temperature.
4. Examples of polymer applications include