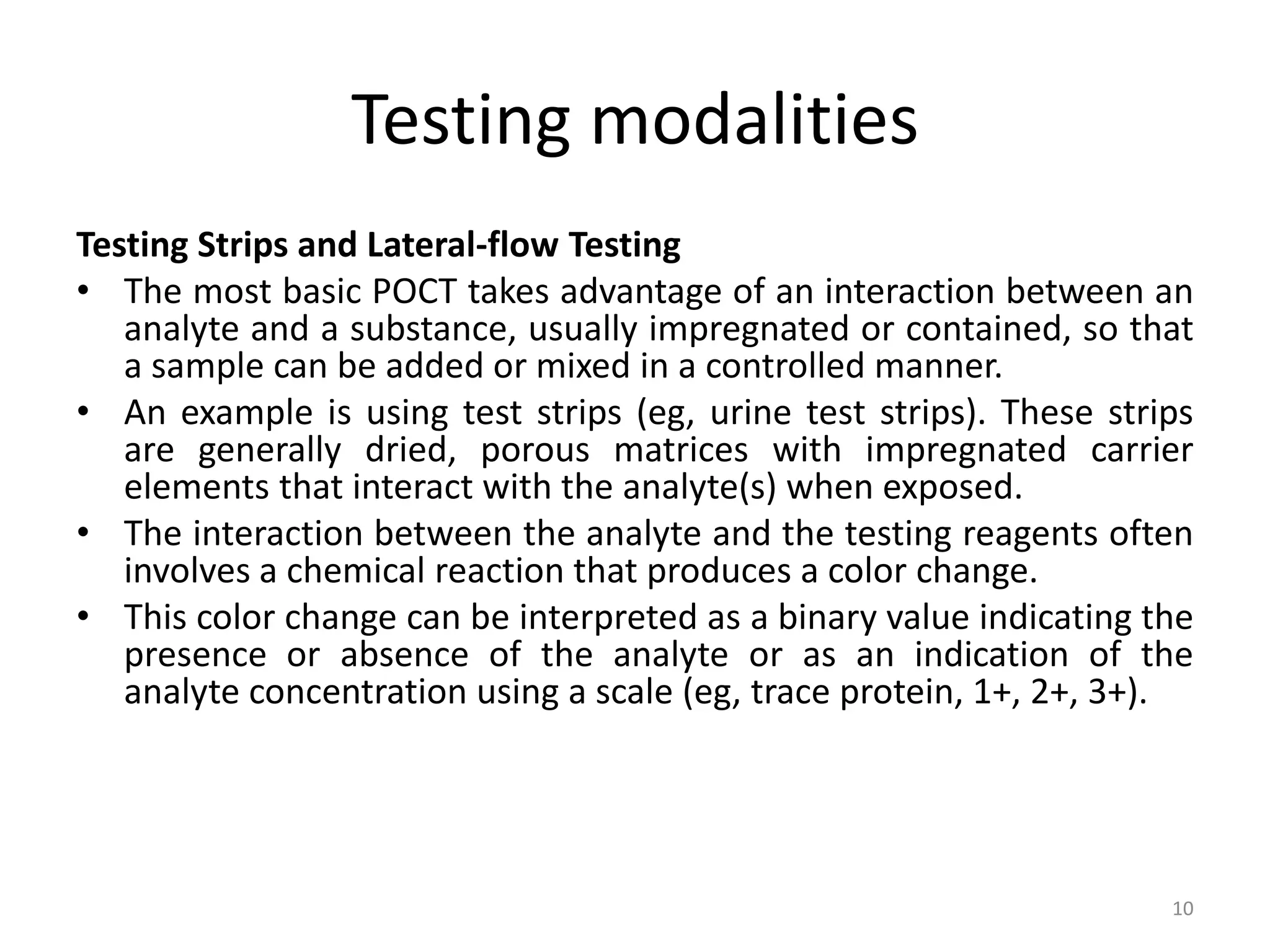
The document outlines the objectives, features, and methodologies of point-of-care testing (POCT), which provides rapid clinical laboratory testing at or near the site of patient care. It emphasizes the importance of quality control, adherence to protocols, and collaboration among healthcare professionals to enhance patient management outcomes. Various testing modalities, including simple test strips and complex immunoassays, are discussed, highlighting their applications, advantages, and limitations in medical diagnostics.










![Immunoassays
• POCT testing that utilizes immunoassays relies
on antibodies to bind to a specific target when
the concentration exceeds a certain
threshold.[14] Targets in immunoassays for
POCT can encompass a wide range of
substances, including proteins, drugs, and
pathogens. POCTs are available in various
formats, including both individual tests and
platforms with multiple built-in tests
14](https://image.slidesharecdn.com/pointofcaretesting-240504143620-135f5845/75/Point-of-Care-Testing-in-selected-laboratory-14-2048.jpg)



