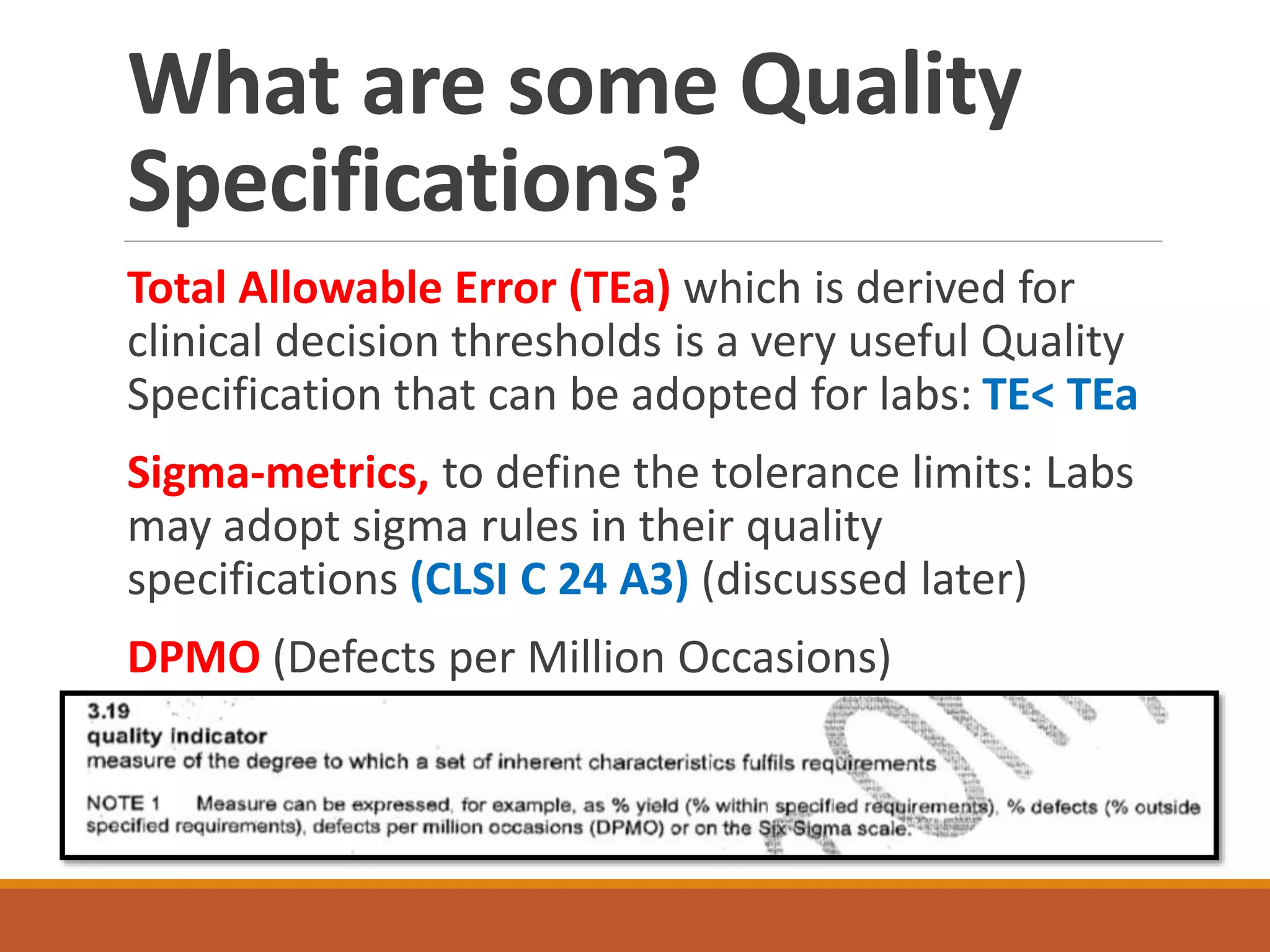
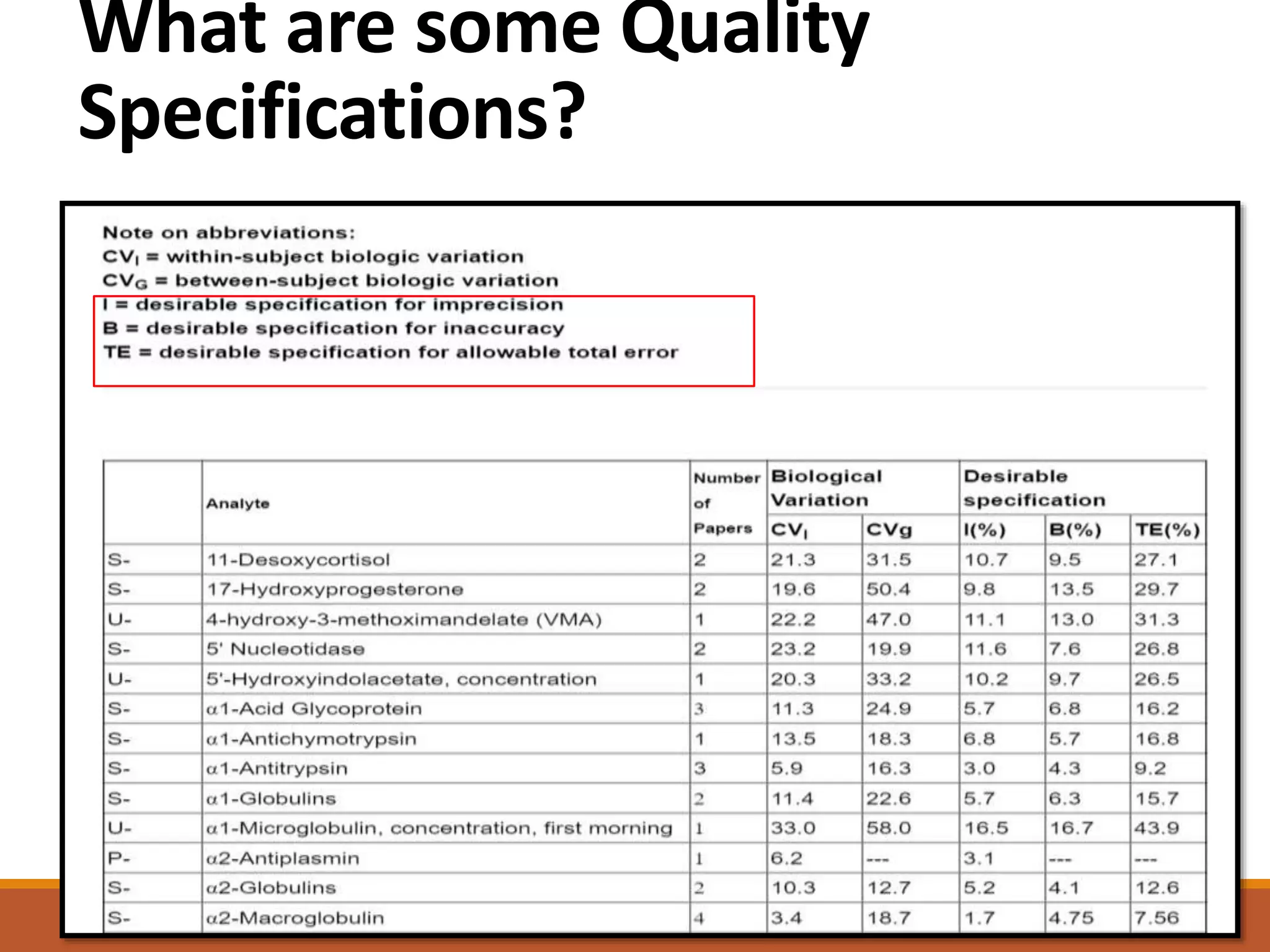
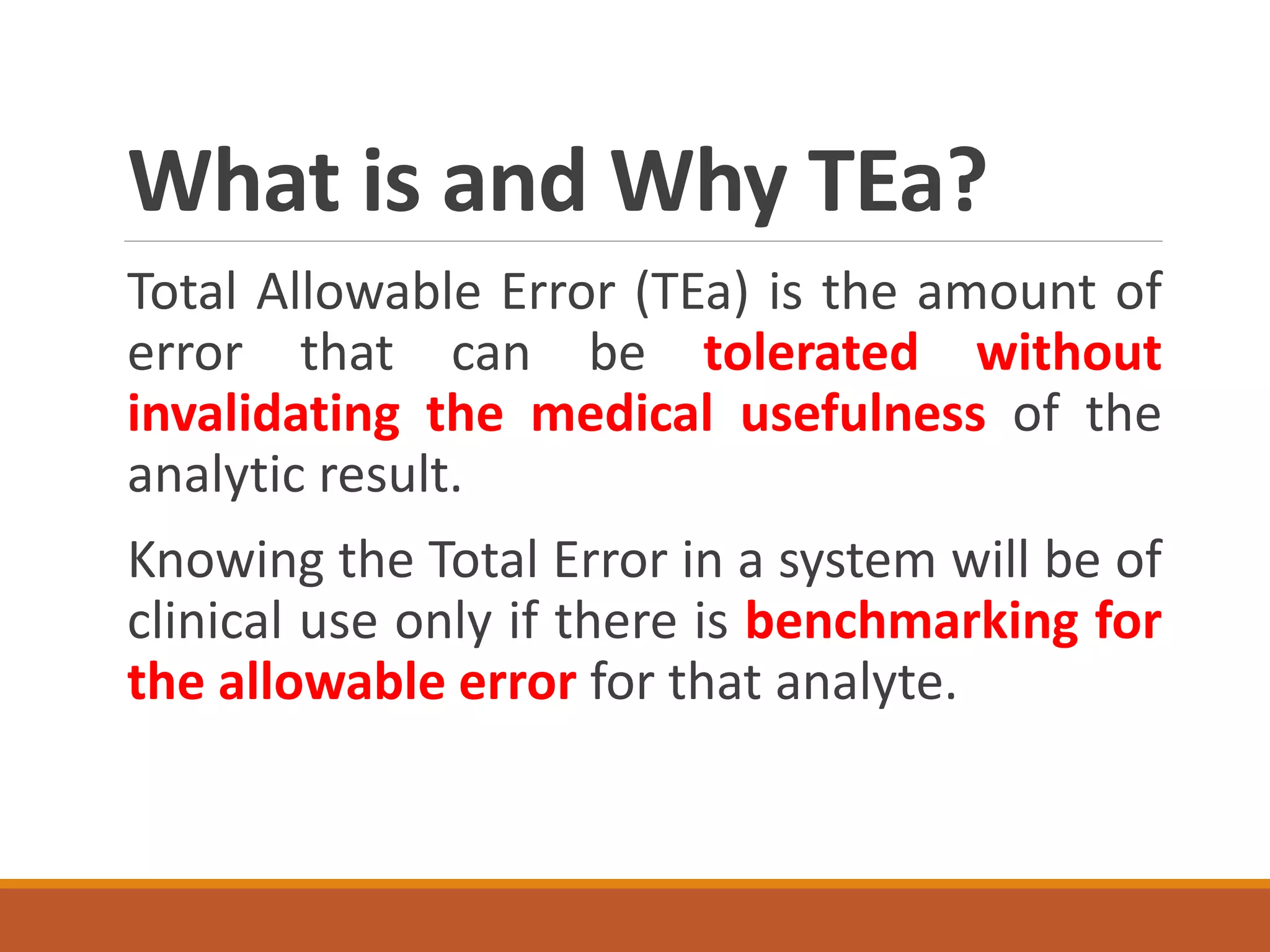
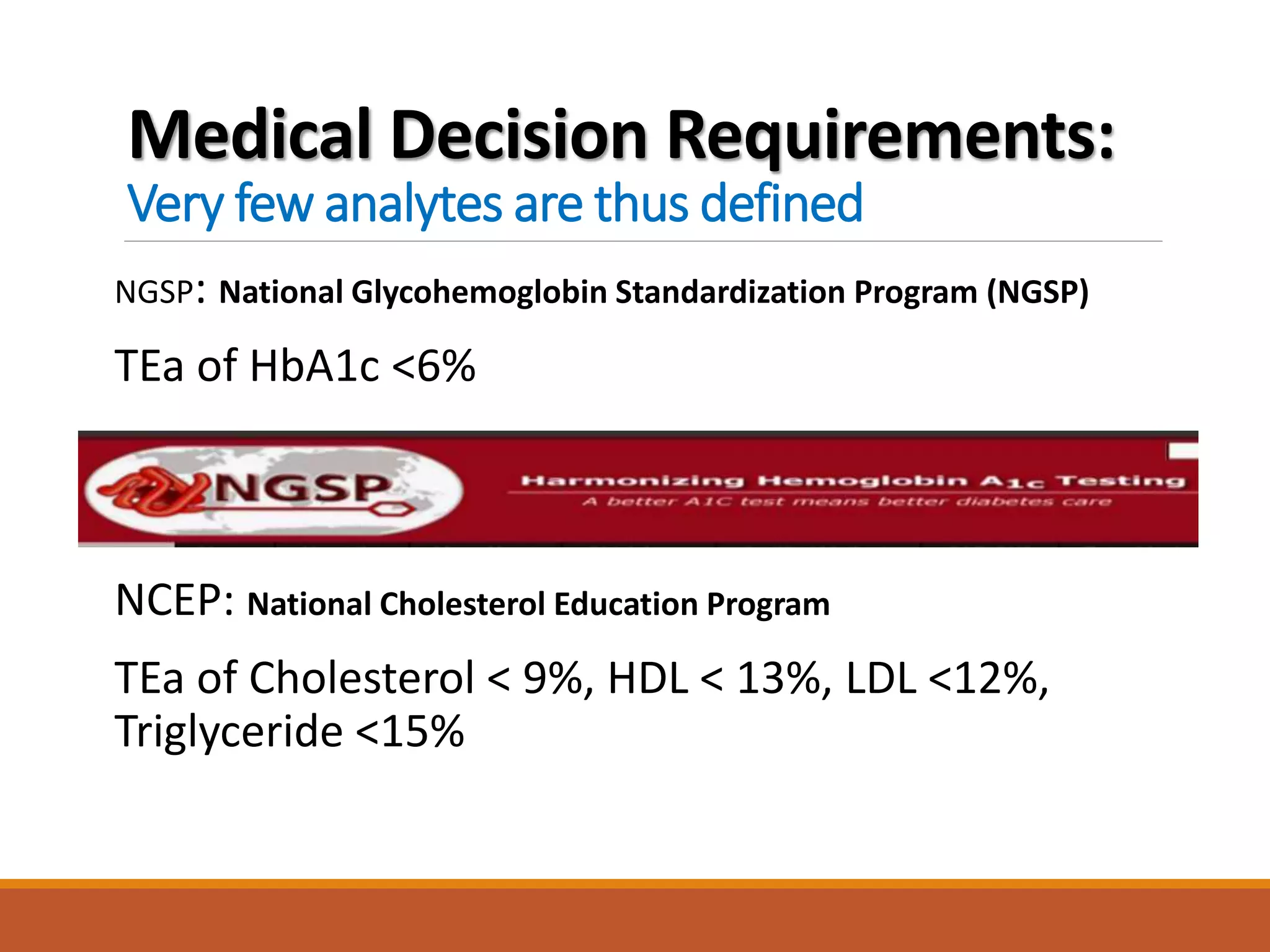
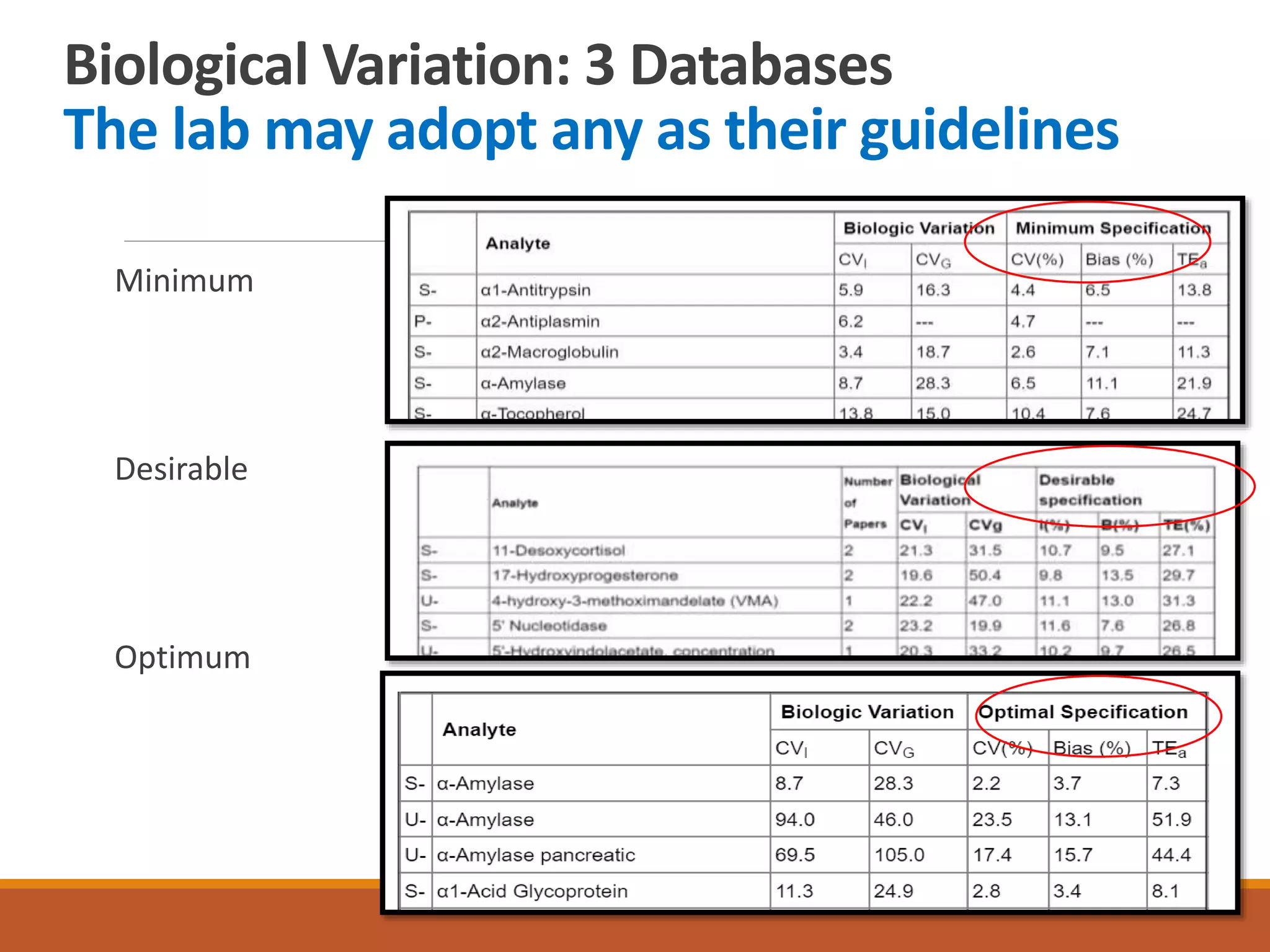
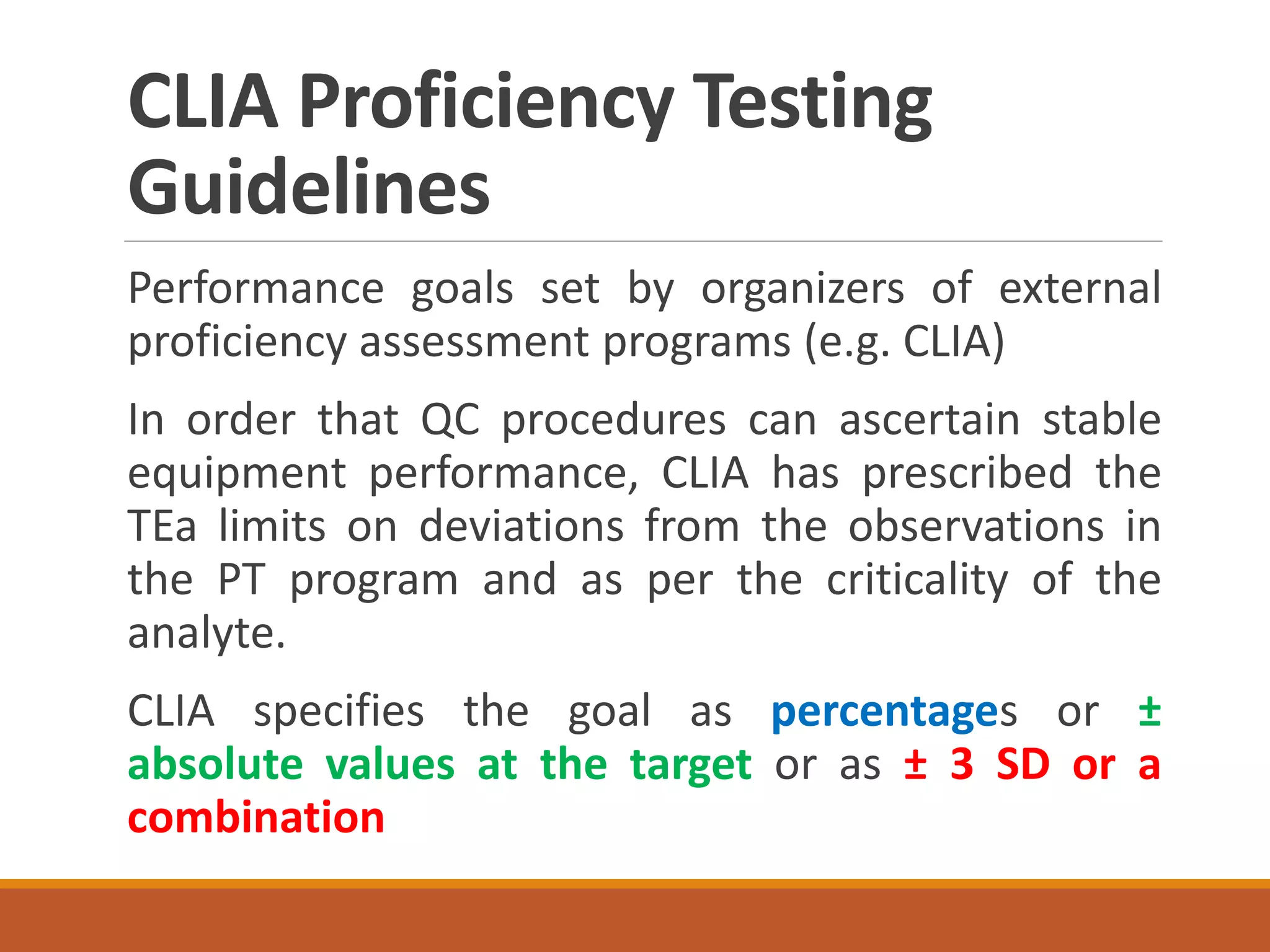
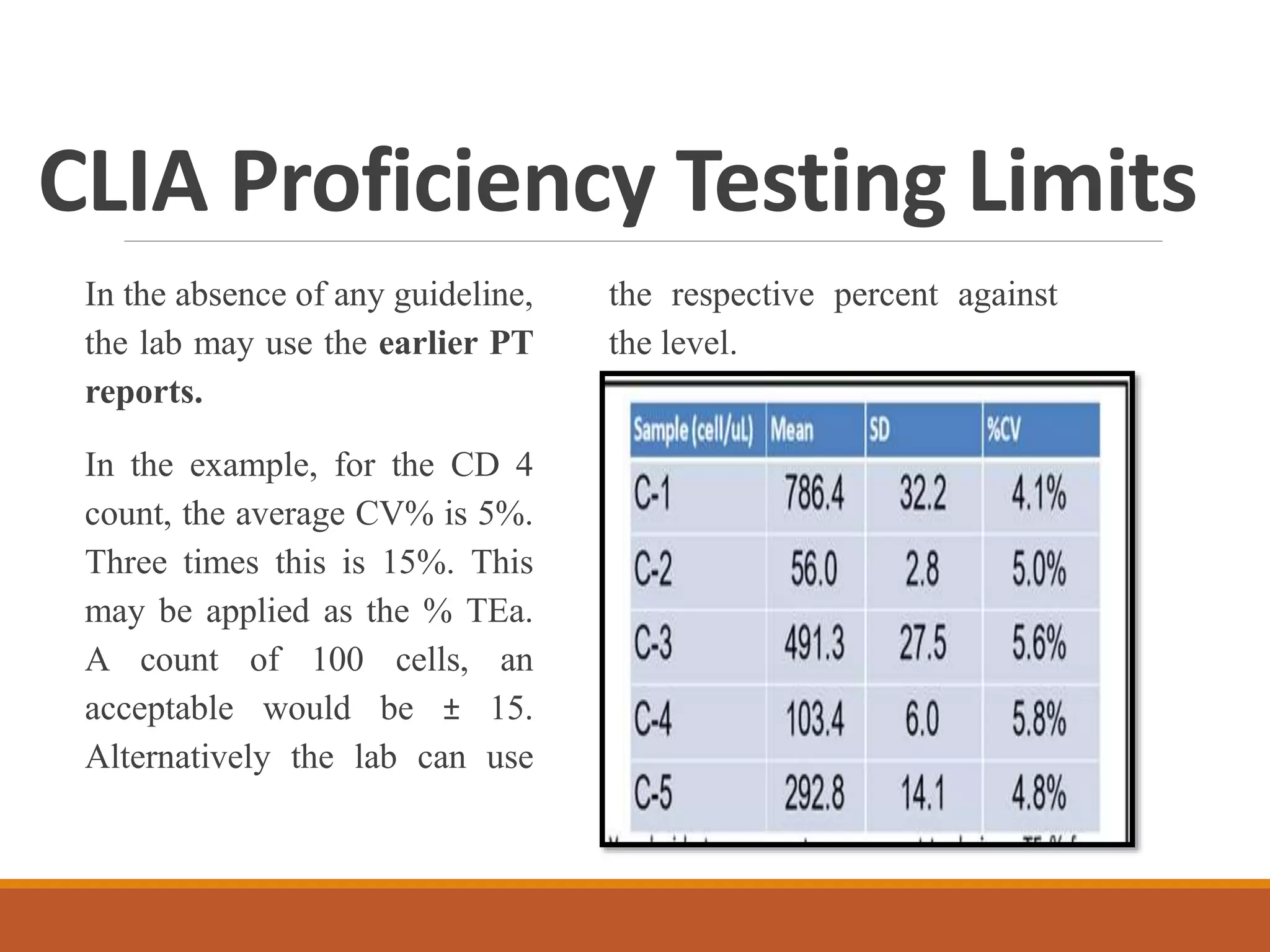
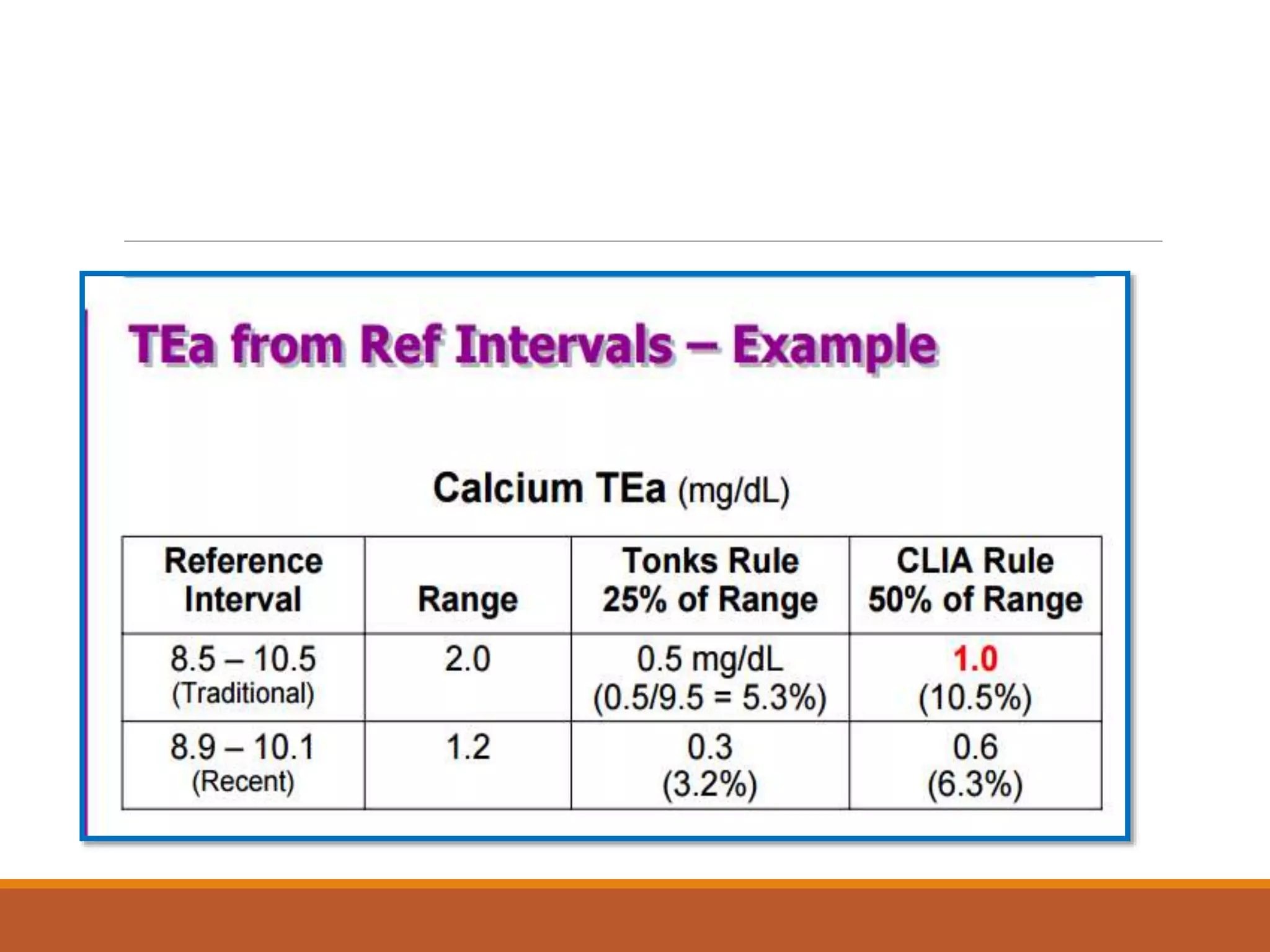
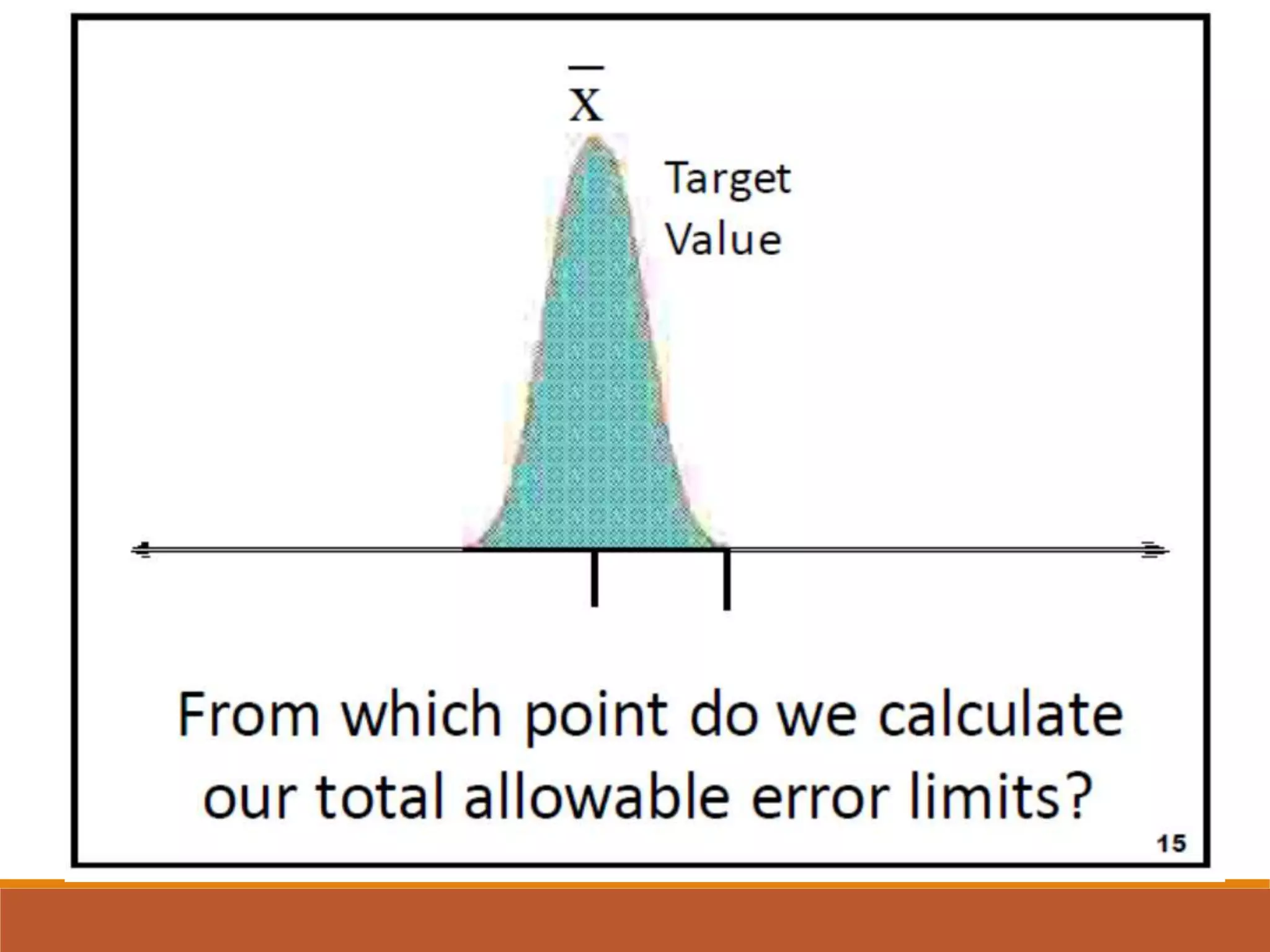
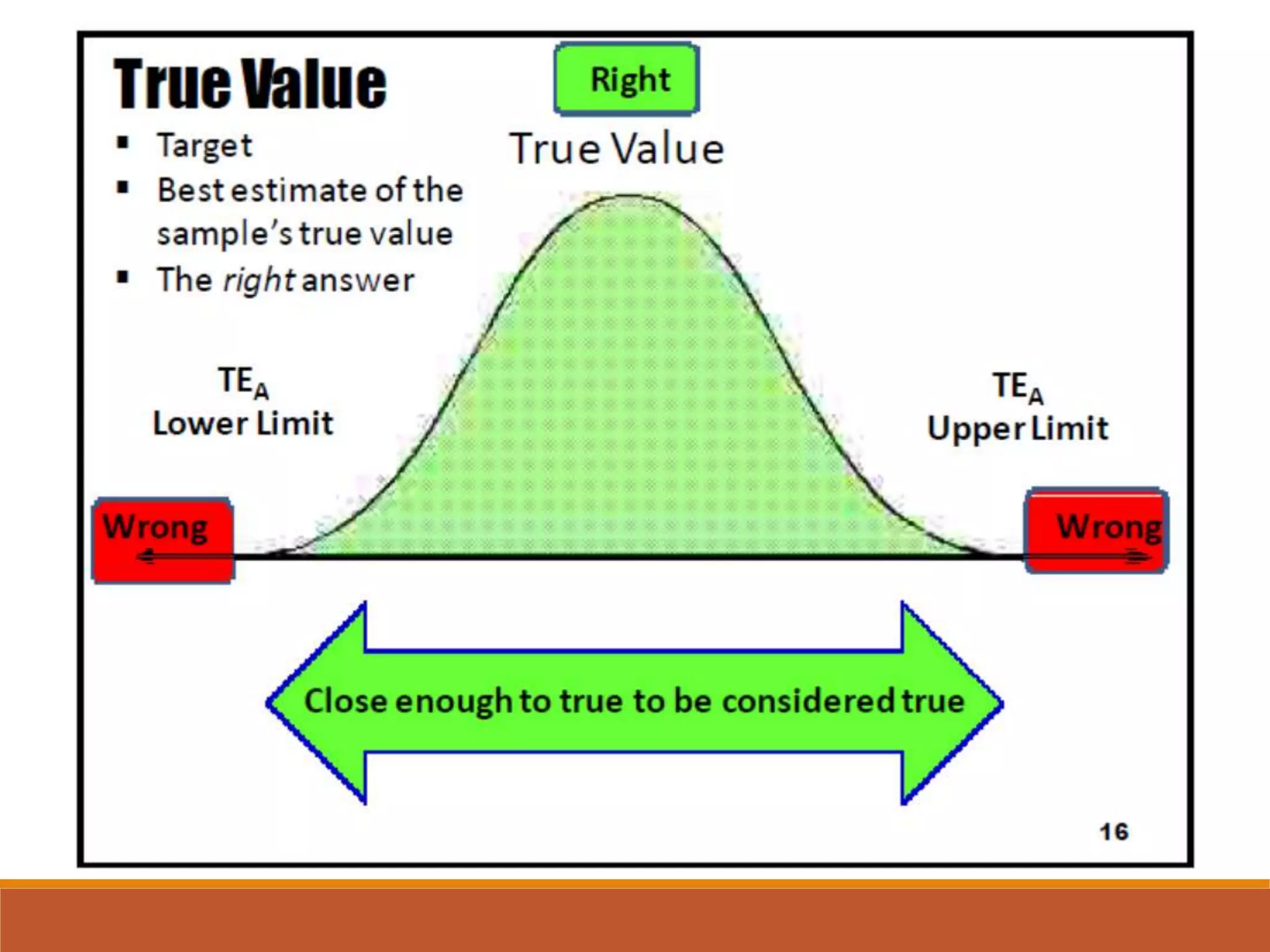
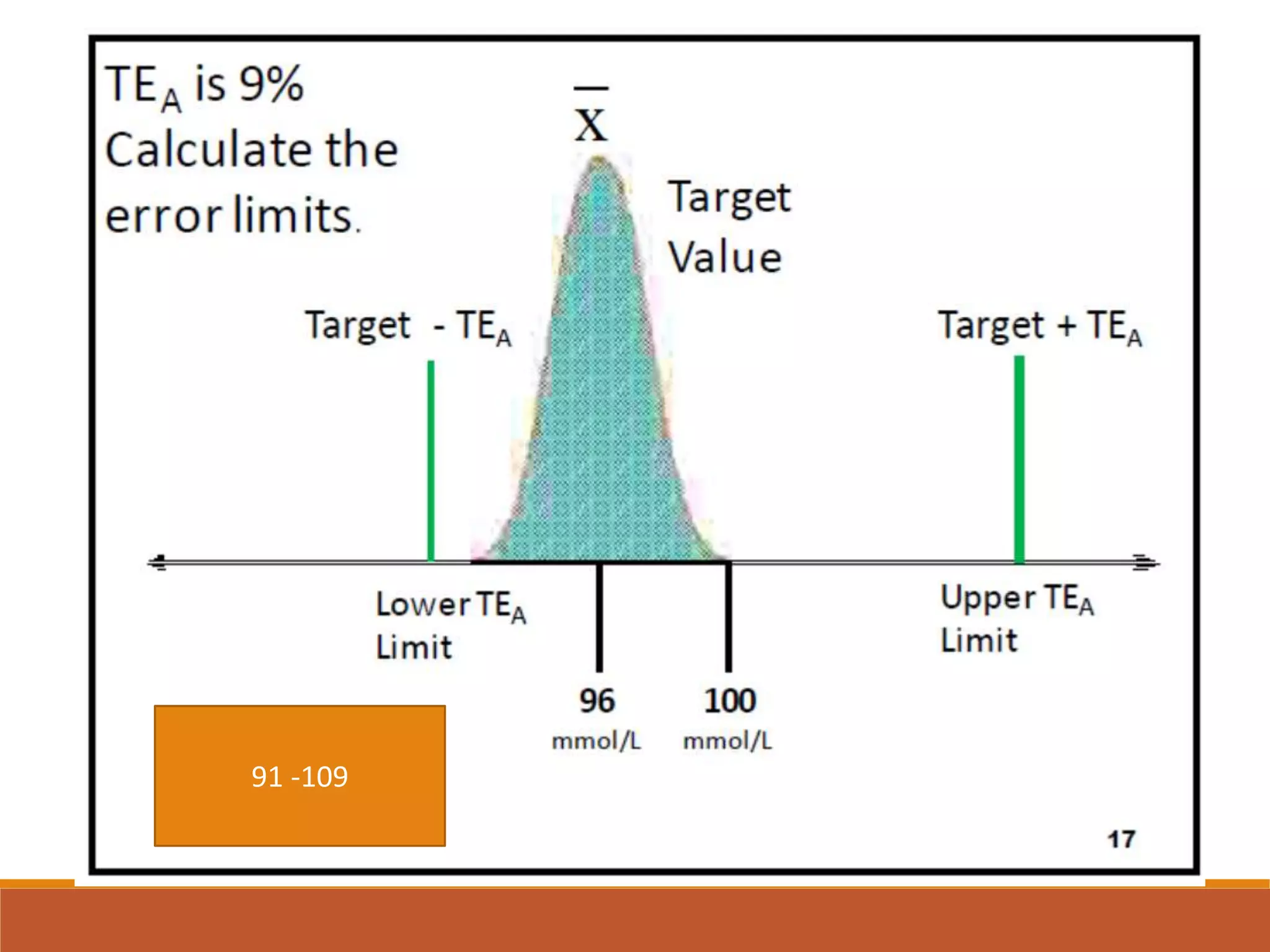
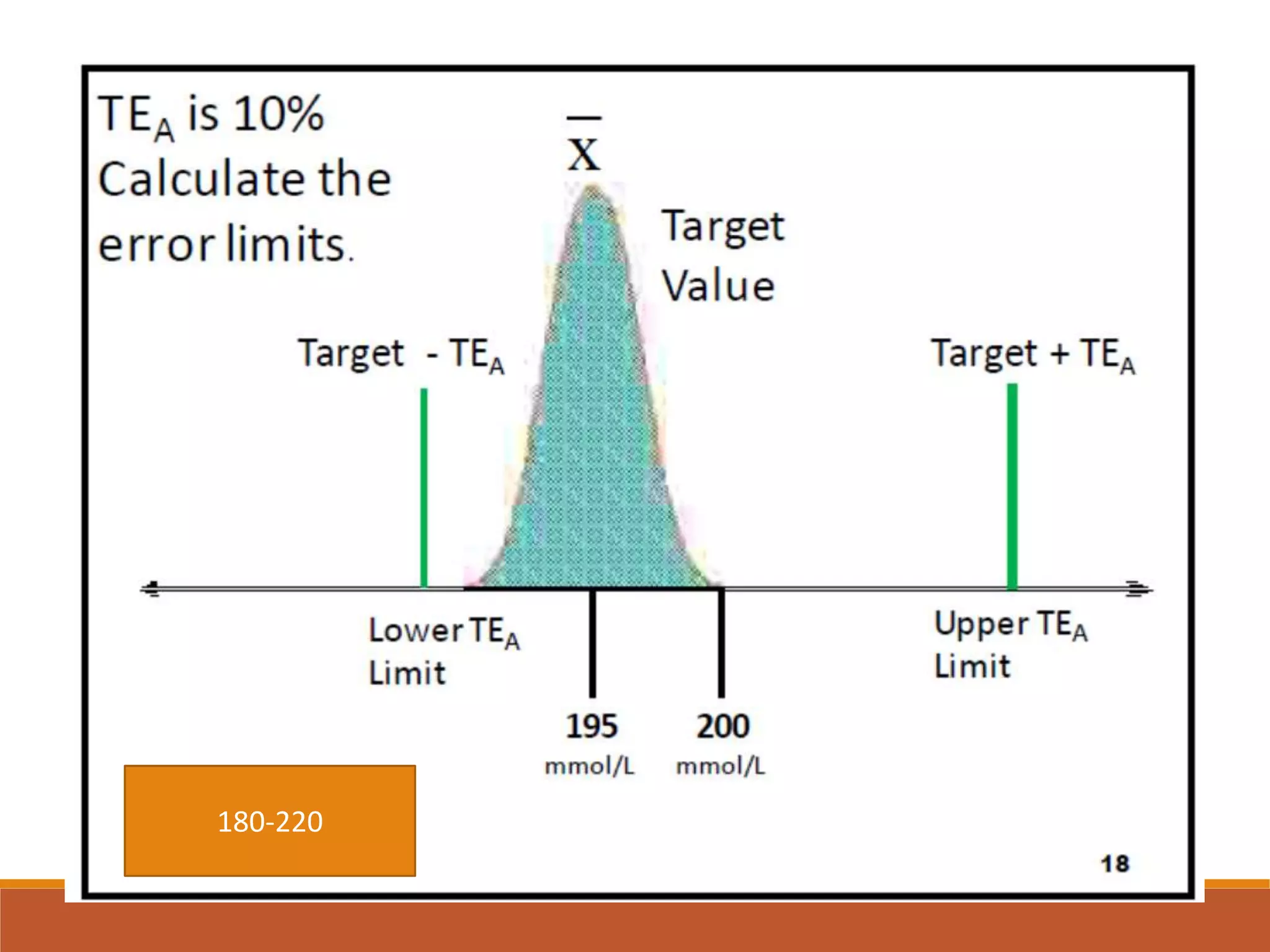
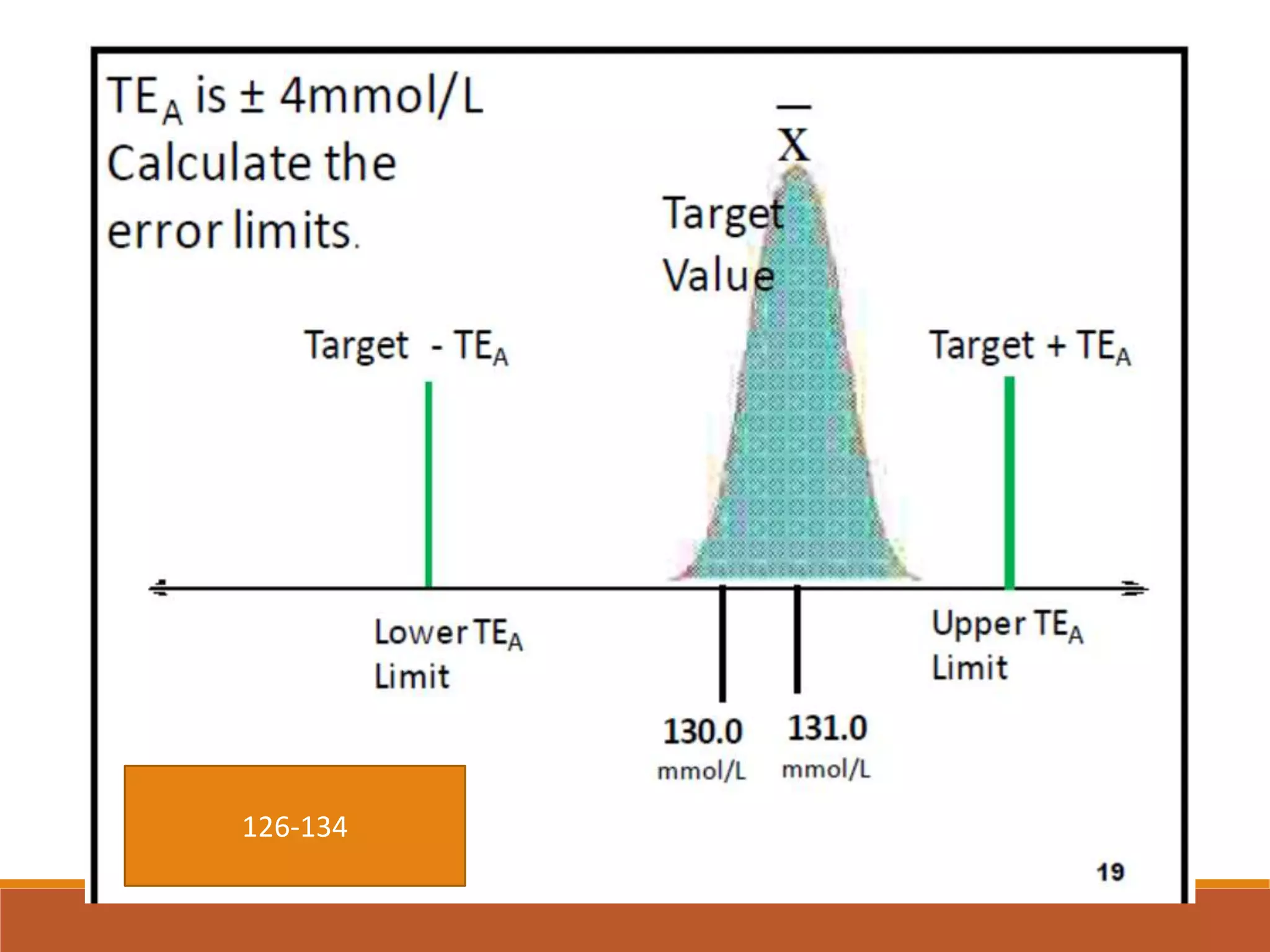
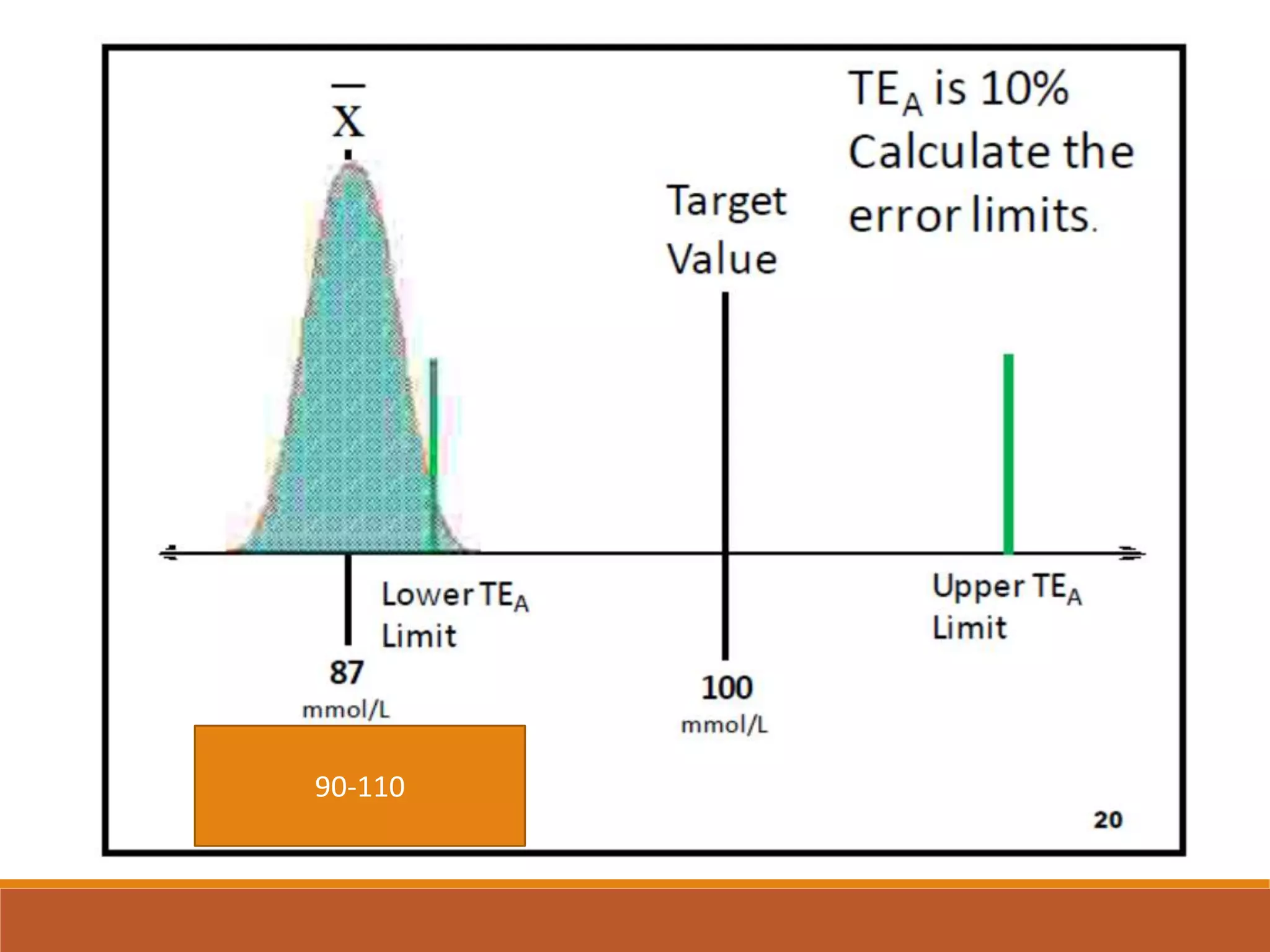
Total Allowable Error (TEa) is a quality specification that provides perspective on test result variability and significance of abnormal findings. TEa values can come from medical decision thresholds, biological variation databases listing within-subject and between-subject variation, or proficiency testing guidelines from CLIA setting performance goals. When no other guidelines exist, labs may use the current running CV multiplied by three as a last resort TEa value. Quality specifications help interpret test results and ensure results remain clinically useful.