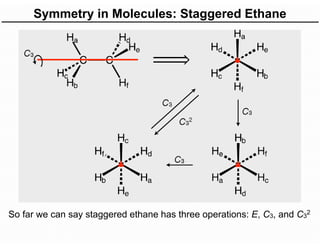
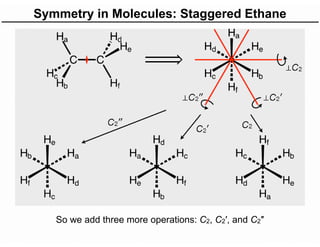
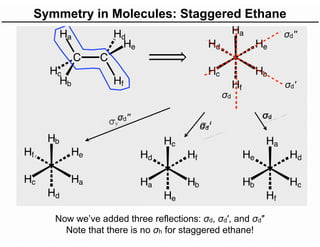
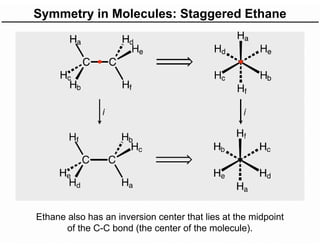
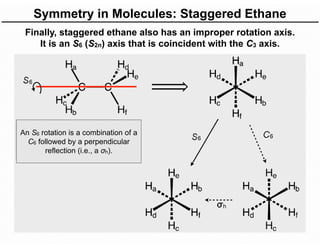
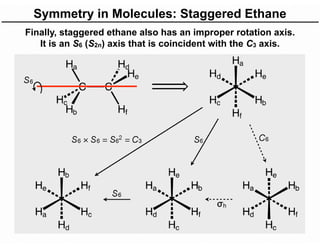
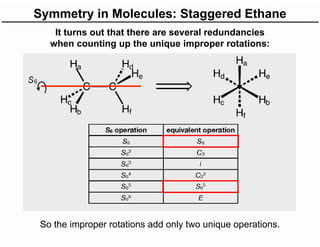
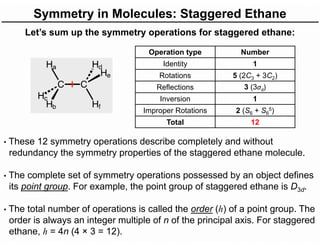
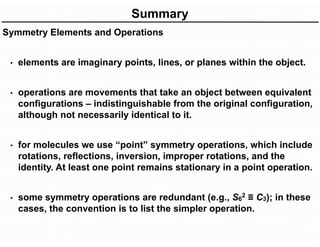
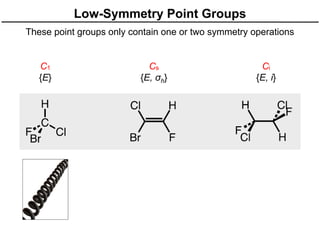
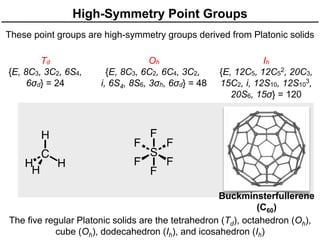
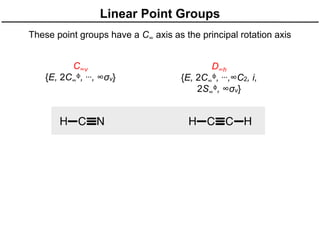
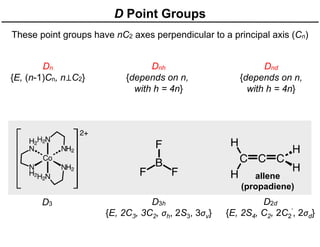
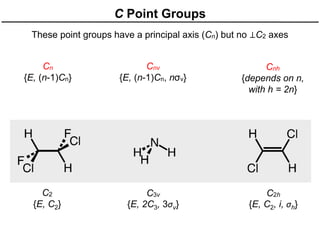
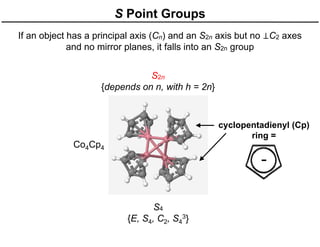
The document discusses the symmetry operations and point group classification of staggered ethane, detailing its specific symmetry elements including rotations, reflections, and improper rotations. It highlights the 12 unique symmetry operations that define the molecule's point group, which is d3d, and explains the importance of symmetry in molecular classification. Additionally, it categorizes various point groups based on their symmetry operations, providing examples of low-symmetry and high-symmetry point groups derived from platonic solids.