The document discusses hemolytic transfusion reactions, including clinical features, workup, and classification. It provides details on:
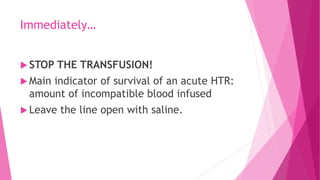
- Immediately stopping the transfusion, clerical checks, and laboratory tests to check for hemolysis like hemoglobinemia, DAT, and LDH.
- Acute hemolytic transfusion reactions within 24 hours can cause fever, hypotension, hemoglobinuria, and are treated with hydration. Delayed reactions over 24 hours are usually milder and extravascular.
- Thorough testing, labeling, and advanced identification methods can help prevent hemolytic transfusion reactions.