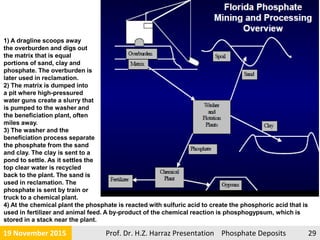
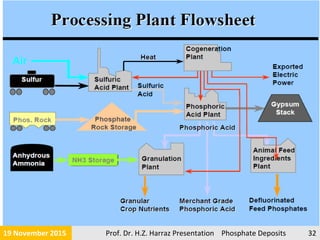
The document presents an extensive overview of phosphorite ore deposits, detailing types such as igneous, biogenic, and sedimentary marine phosphate deposits, with a focus on their occurrence, mineral composition, and economic significance. Phosphorite is defined as a sedimentary rock with at least 15-20% phosphate, primarily used for various commercial applications and formed under specific geological processes. The global production landscape highlights major producers and the economic relevance of phosphate resources, emphasizing the need for efficient extraction methods and understanding of marine depositional environments.