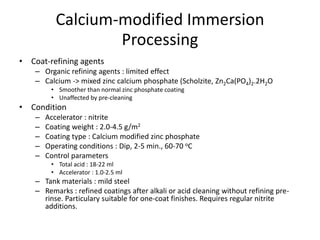
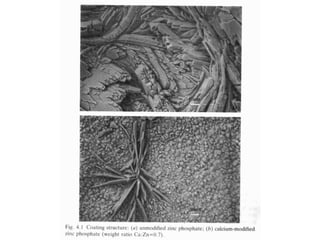
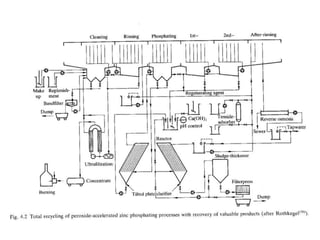
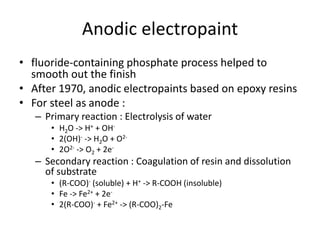
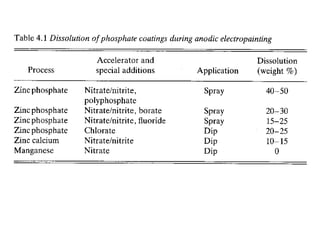
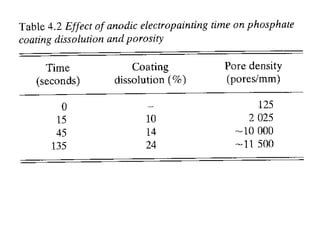
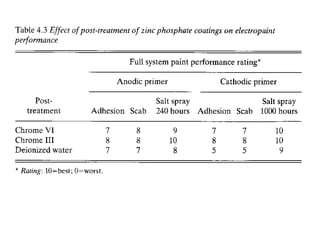
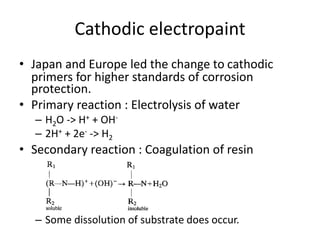
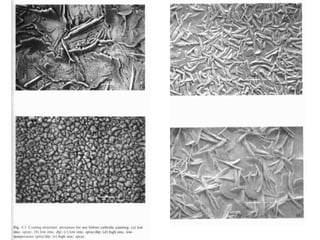
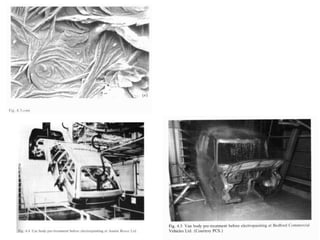
This document discusses different methods of phosphating ferrous and aluminum alloys as a pre-treatment for paint bonding, including immersion zinc phosphate processes, spray zinc phosphate processes, and electropaint processes. It provides details on the chemistry, operating conditions, and advantages/disadvantages of orthodox immersion processing, calcium-modified immersion processing, and various spray phosphate processes. It also compares anodic and cathodic electropaint systems and their performance. Phosphate coatings formed by immersion are generally superior to sprays as a pre-treatment for electropaint.