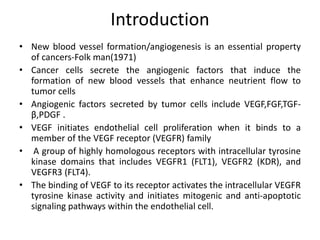
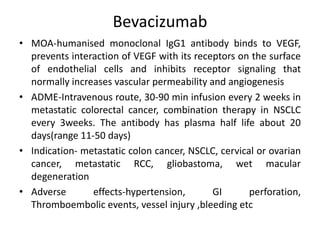
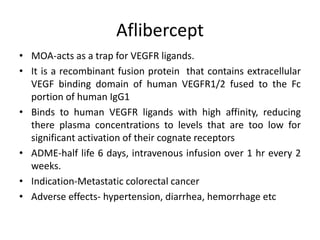
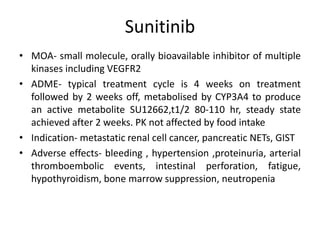
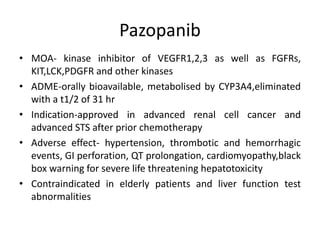
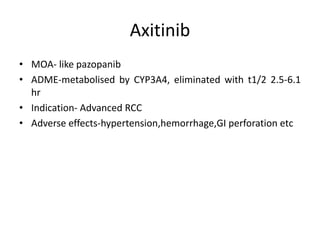
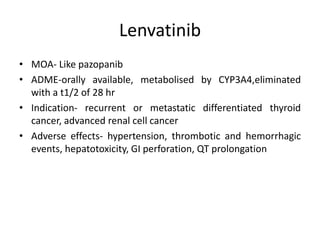
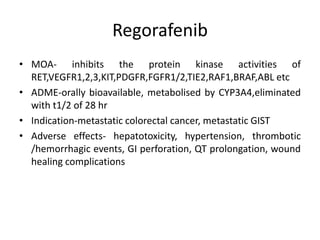
Anti-VEGF drugs and HDAC inhibitors are used in cancer treatment. Anti-VEGF drugs inhibit VEGF signaling by blocking the VEGF ligand or receptor and include monoclonal antibodies like bevacizumab and small molecule inhibitors like sunitinib. They are used to treat cancers like colorectal, lung, and kidney cancer. HDAC inhibitors increase histone acetylation, activate tumor suppressor genes, and induce cell cycle arrest. Panobinostat, romidepsin, and vorinostat are examples used for blood cancers and lymphomas. Both classes of drugs can cause side effects like hypertension, bleeding, and fatigue.









![References
1.Goodman & Gilman’s the pharmacological basis of
therapeutics 13th edition
2.Bolden, J.E.; Peart, M.M.J.; Johnstone, R.R.W. Anticancer
activities of histone deacetylase inhibitors. Nat. Rev.Drug
Discov. 2006, 5, 769–784. [CrossRef] [PubMed]
3. Butler LM, Zhou X, Xu WS, Scher HI, Rifkind RA, MarksPA et al.
(2002).The histone deacetylase inhibitor SAHA arrests cancer
cell growth, up-regulates thioredoxin-bindingprotein-2, and
down-regulates thioredoxin. Proc Natl AcadSci USA 99:
11700–11705.](https://image.slidesharecdn.com/vegfinhibitors-211029154345/85/Vegf-inhibitors-22-320.jpg)
