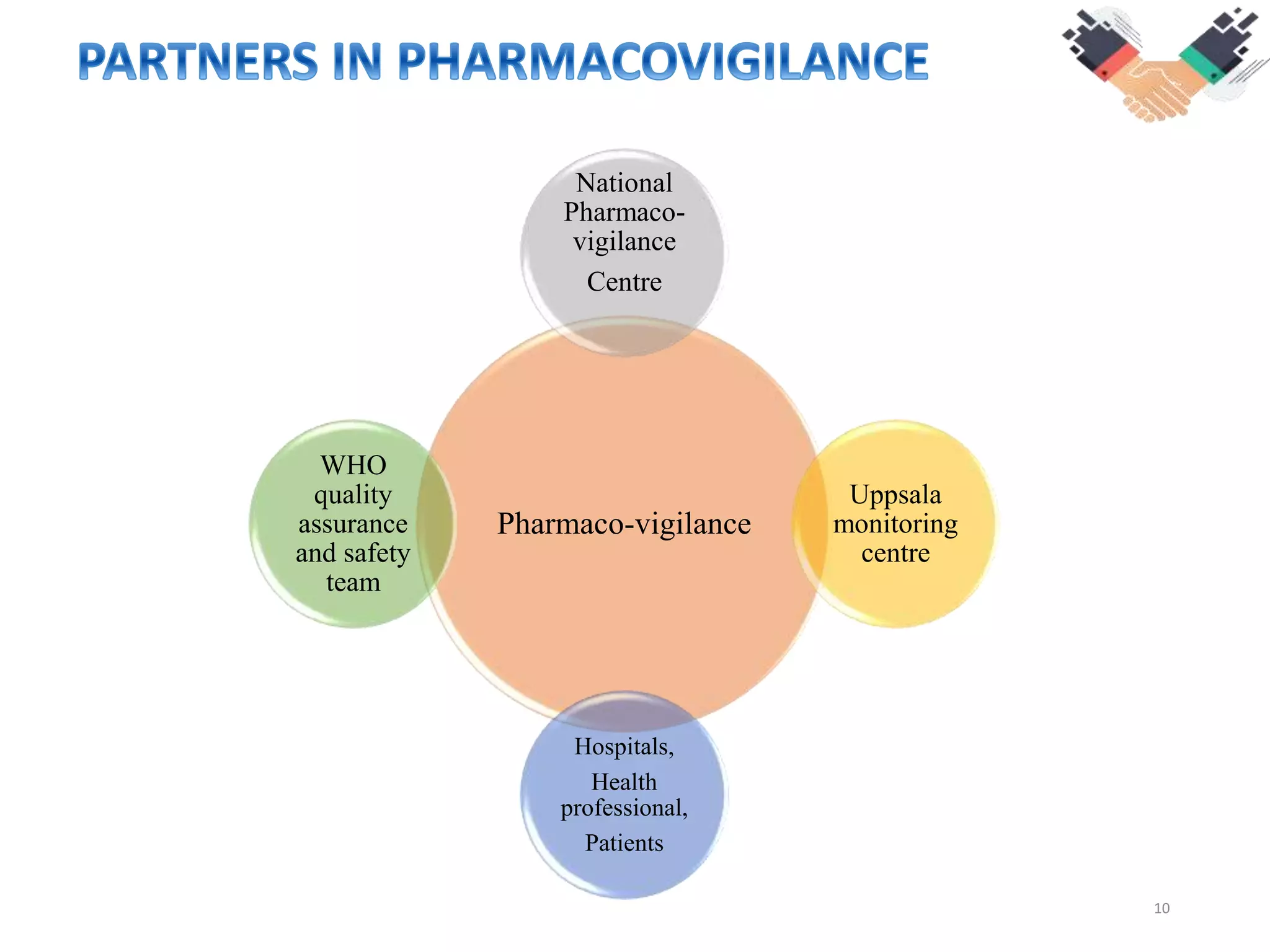
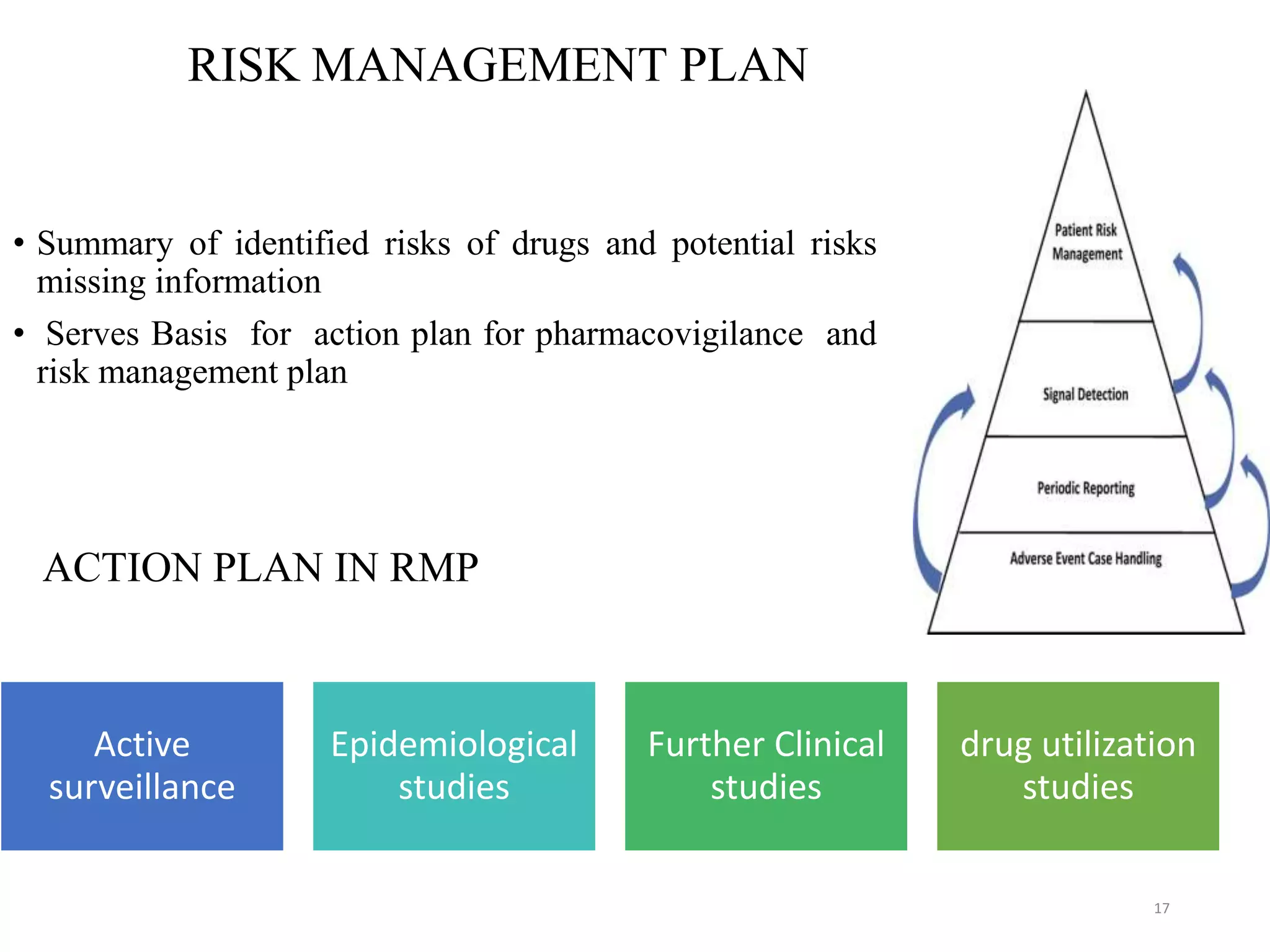
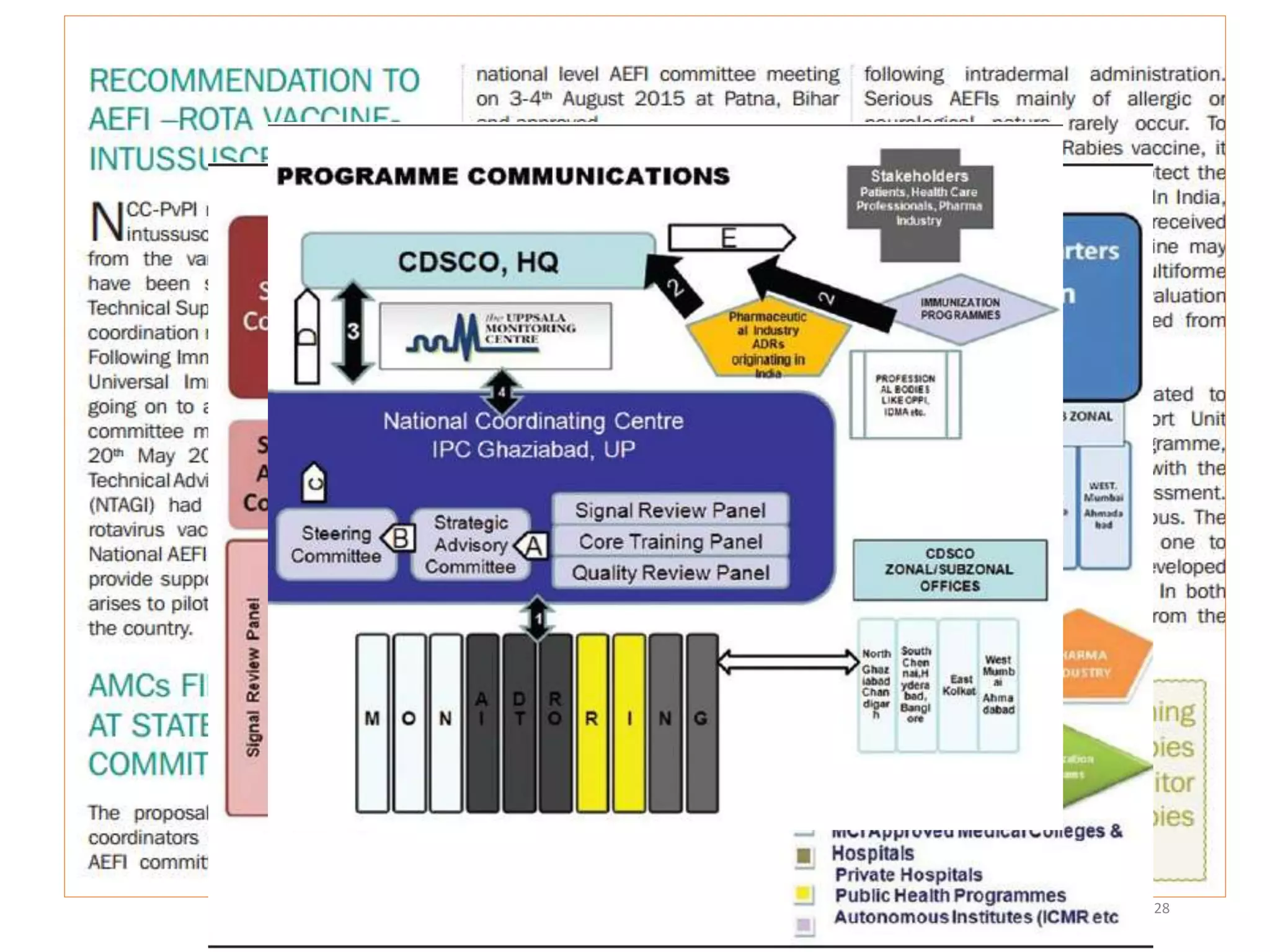
This document discusses pharmacovigilance and the need for monitoring drug safety post approval. It describes how historical drug safety issues like the Elixir Sulfanilamide and Thalidomide tragedies revealed limitations in pre-approval testing and established the need for ongoing pharmacovigilance. The aims, application and reporting processes of pharmacovigilance are outlined along with terminology and examples of regulatory actions taken based on adverse event reporting.