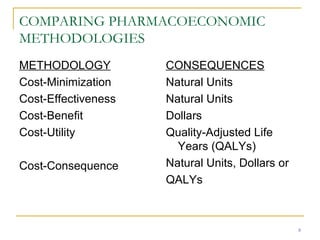
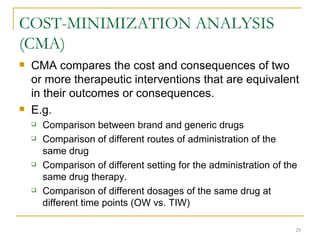
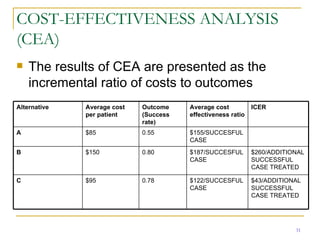
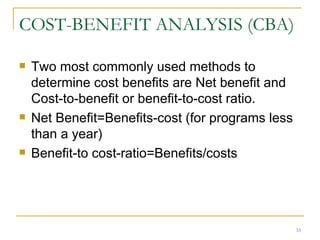
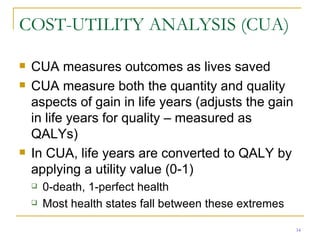
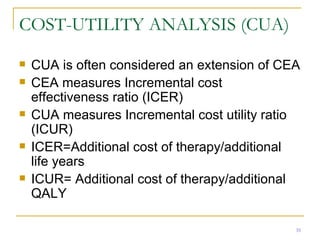
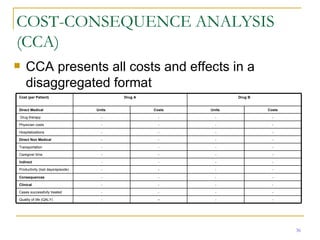
This document provides an overview of the pharmacoeconomics research process. It discusses the uses and need for pharmacoeconomics research, as well as the main types of analyses including cost-minimization analysis, cost-effectiveness analysis, cost-benefit analysis, cost-utility analysis, and cost-consequence analysis. The key steps in conducting a pharmacoeconomic evaluation are defined such as identifying the problem, alternative interventions, outcomes and costs. Sensitivity analysis and discounting are also summarized.