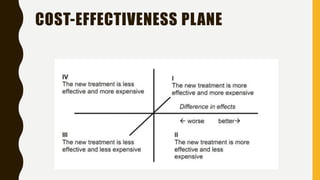
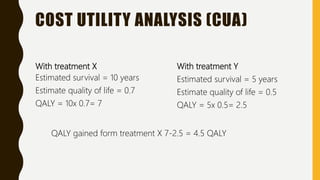
This document discusses pharmacoeconomics, which compares the value of drug therapies. It defines pharmacoeconomics and explains why it is important given rising healthcare costs. The key methods of pharmacoeconomic analysis include cost of illness, cost-minimization analysis, cost-benefit analysis, cost-effectiveness analysis, and cost-utility analysis. These methods are used to evaluate costs and consequences of drug therapies from various perspectives to inform healthcare policy and clinical decisions. Limitations of pharmacoeconomics include potential for bias as studies are often funded by interested pharmaceutical companies.