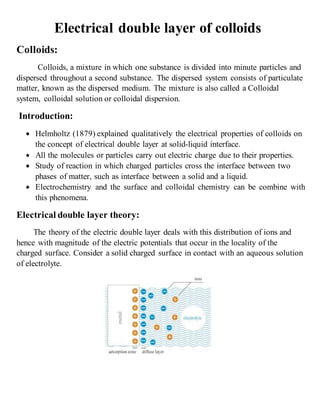
The electrical double layer theory deals with the distribution of ions that occurs at the interface between a charged solid surface (such as a colloid) in contact with an aqueous electrolyte solution. The theory proposes that ions of the opposite charge are attracted to the solid surface (known as the potential determining ions), forming a tightly bound layer, giving the surface a net positive or negative charge. Nearby, counter ions of the opposite charge form a diffuse layer in the solution. The difference in electric potential between the charged surface and the neutral point in the diffuse layer is known as the Nernst potential. The electrical double layer consists of a fixed Stern layer near the surface and a diffuse layer extending into the solution.