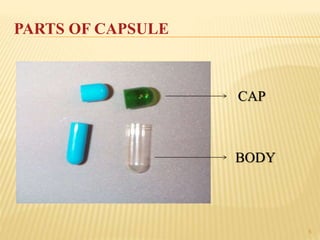
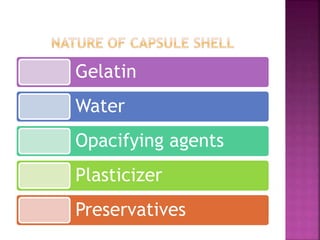
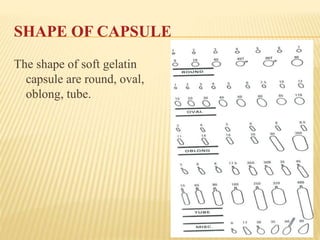
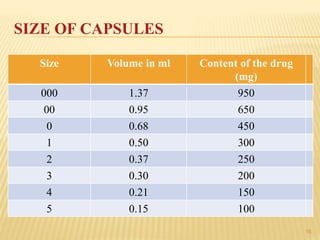
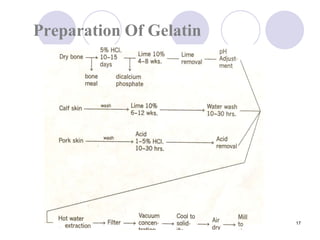
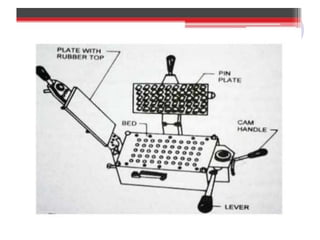
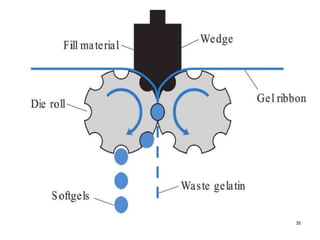
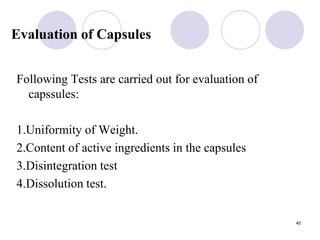
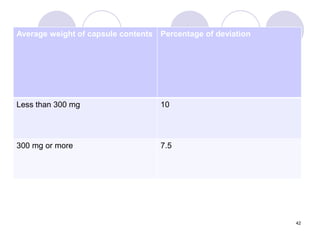
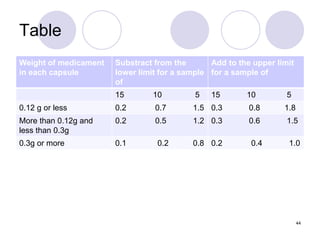
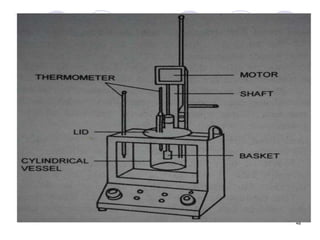
The document discusses the characteristics and preparation of hard and soft gelatin capsules, detailing their composition, manufacturing processes, advantages, and disadvantages. It covers types of gelatin, capsule filling methods, capsule sizes, and the evaluation tests necessary for quality control. Key aspects include the ratio of plasticizers, flow properties of powders, and specifications for capsule assays and dissolution tests.