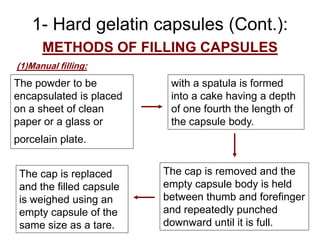
Capsules are solid dosage forms containing powder, liquid, or semisolid medications enclosed in gelatin shells. There are two main types: hard gelatin capsules consisting of two gelatin pieces filled manually or mechanically, and soft gelatin capsules formed, filled, and sealed as a continuous gelatin shell around a liquid core. Capsules offer advantages like taste masking and flexibility in dosages but have limitations for certain active ingredients. Quality is ensured through tests of content uniformity, weight uniformity, disintegration, and dissolution. Enteric coating and sustained release systems allow targeted drug delivery.