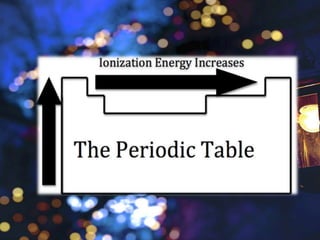
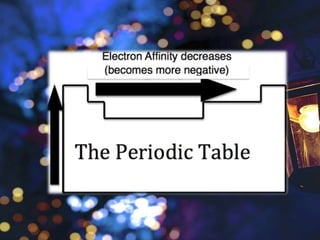
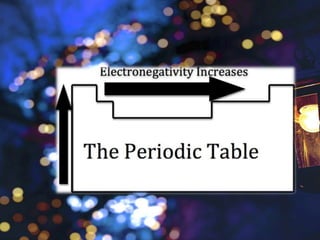
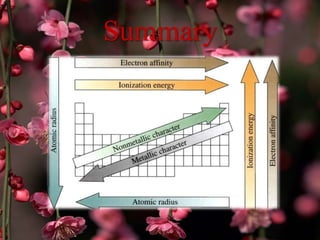
The document discusses the periodic properties of elements, focusing on atomic radius, ionization energy, electron affinity, electronegativity, and metallic character. It describes trends in these properties across the periodic table, highlighting how atomic radius generally increases down a group and decreases across a period, and how ionization energy and electronegativity typically increase from left to right and bottom to top. The document emphasizes the distinction between metals and nonmetals in terms of their tendency to lose or gain electrons.