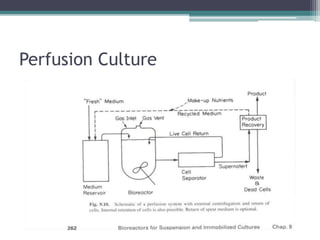
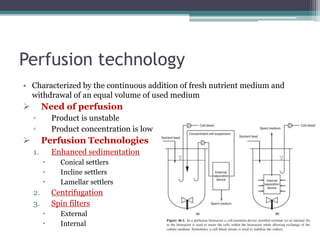
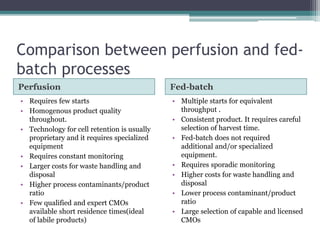
This document discusses perfusion culture systems. It begins by defining perfusion culture as a system where waste medium is continuously removed from the culture and replaced with fresh medium while retaining viable cells. It then discusses advantages of perfusion culture like high cell density, productivity and flexibility. It also covers cell retention methods in perfusion bioreactors like alternating tangential flow filtration and centrifugation. The document concludes by noting that perfusion culture can offer benefits like improved efficiency but requires consideration of validation and regulatory issues.