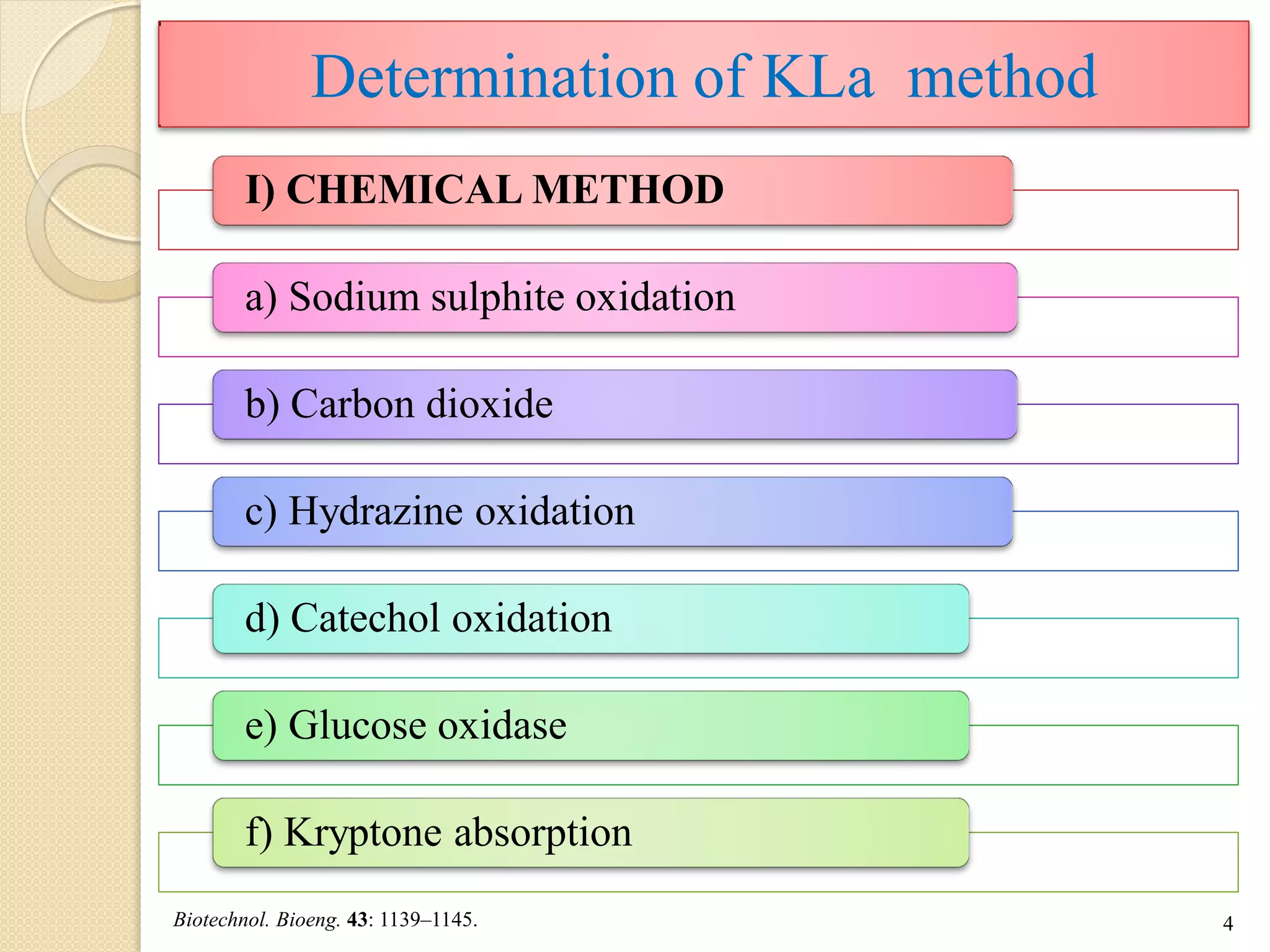
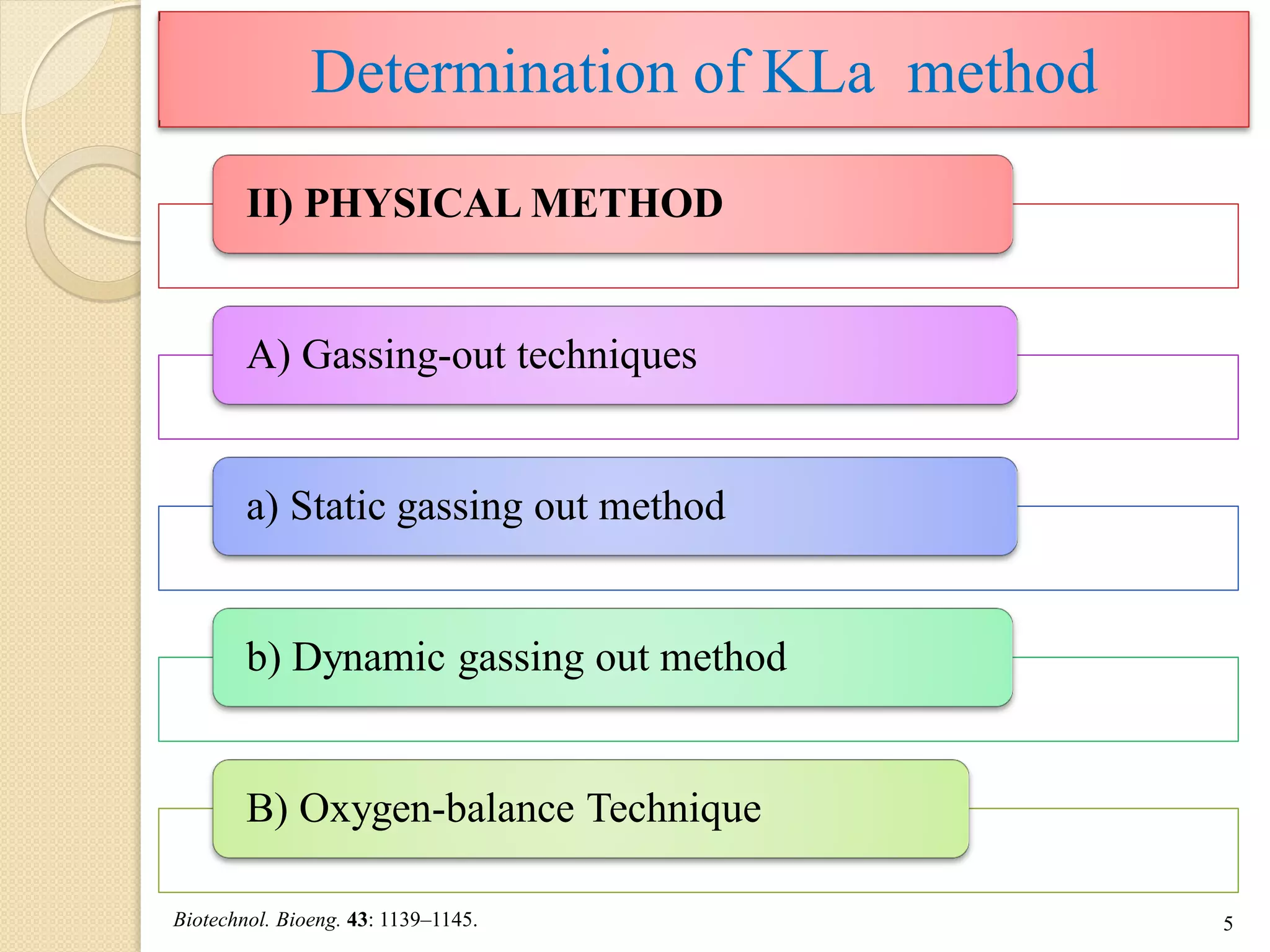
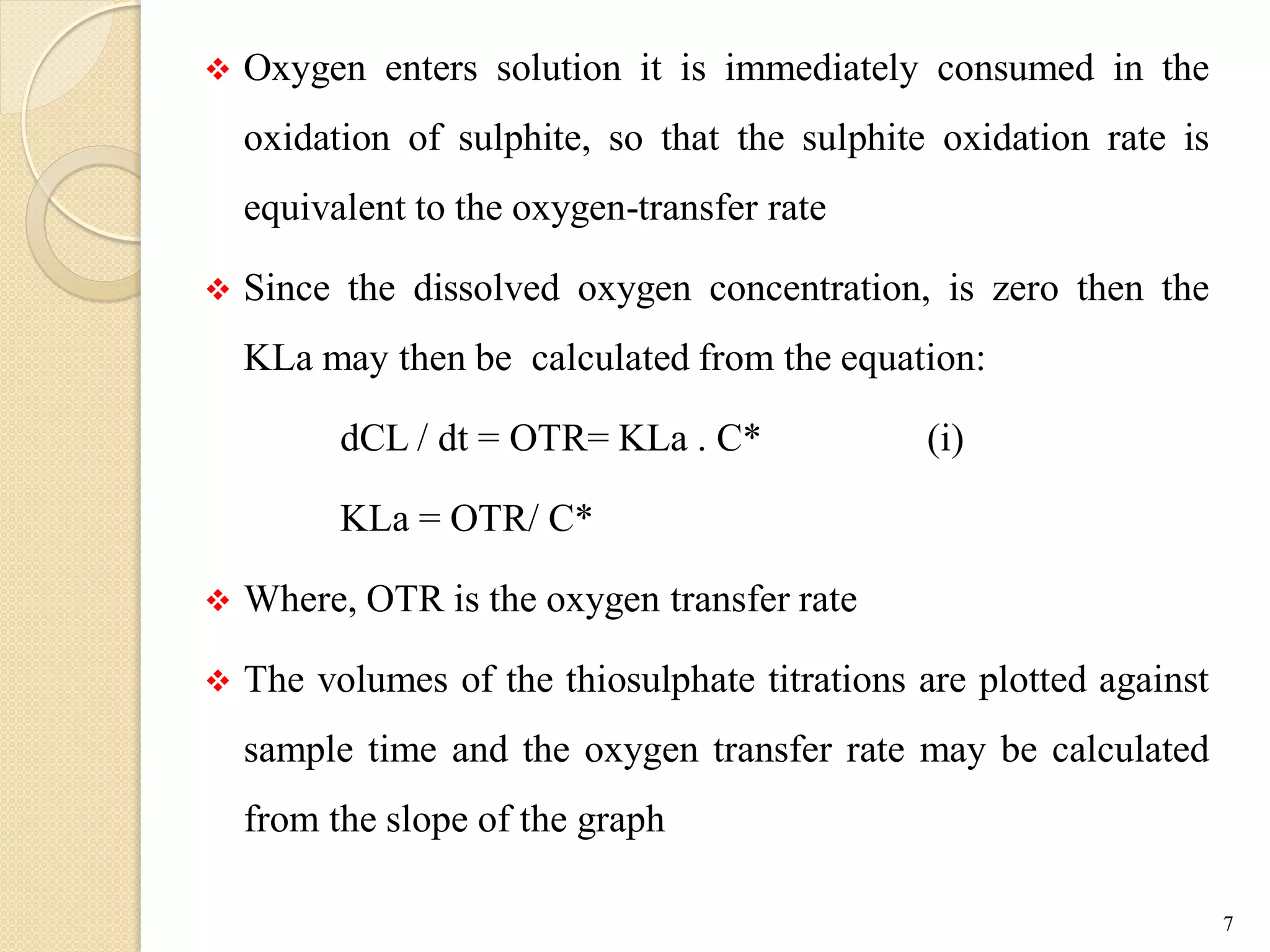
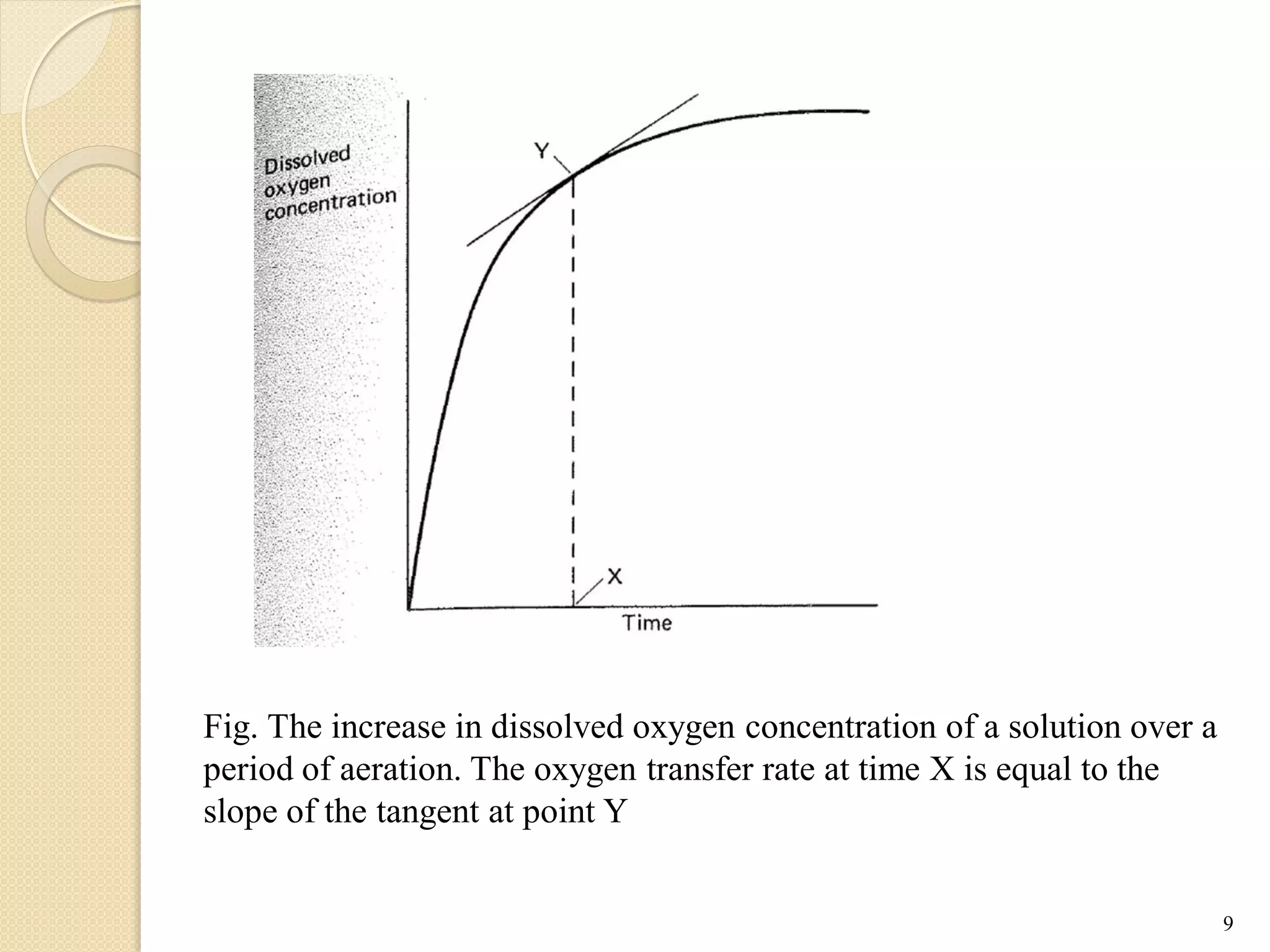
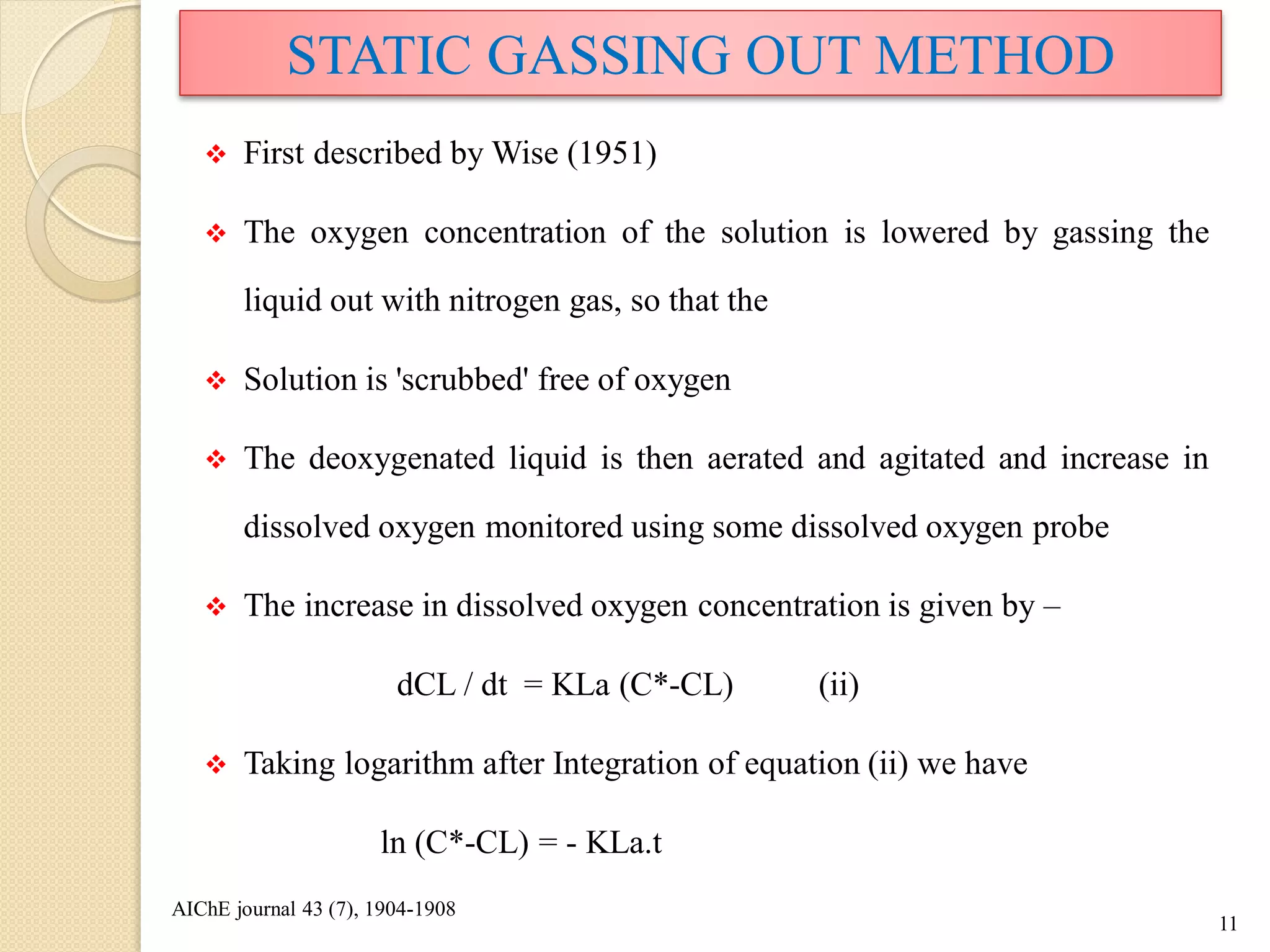
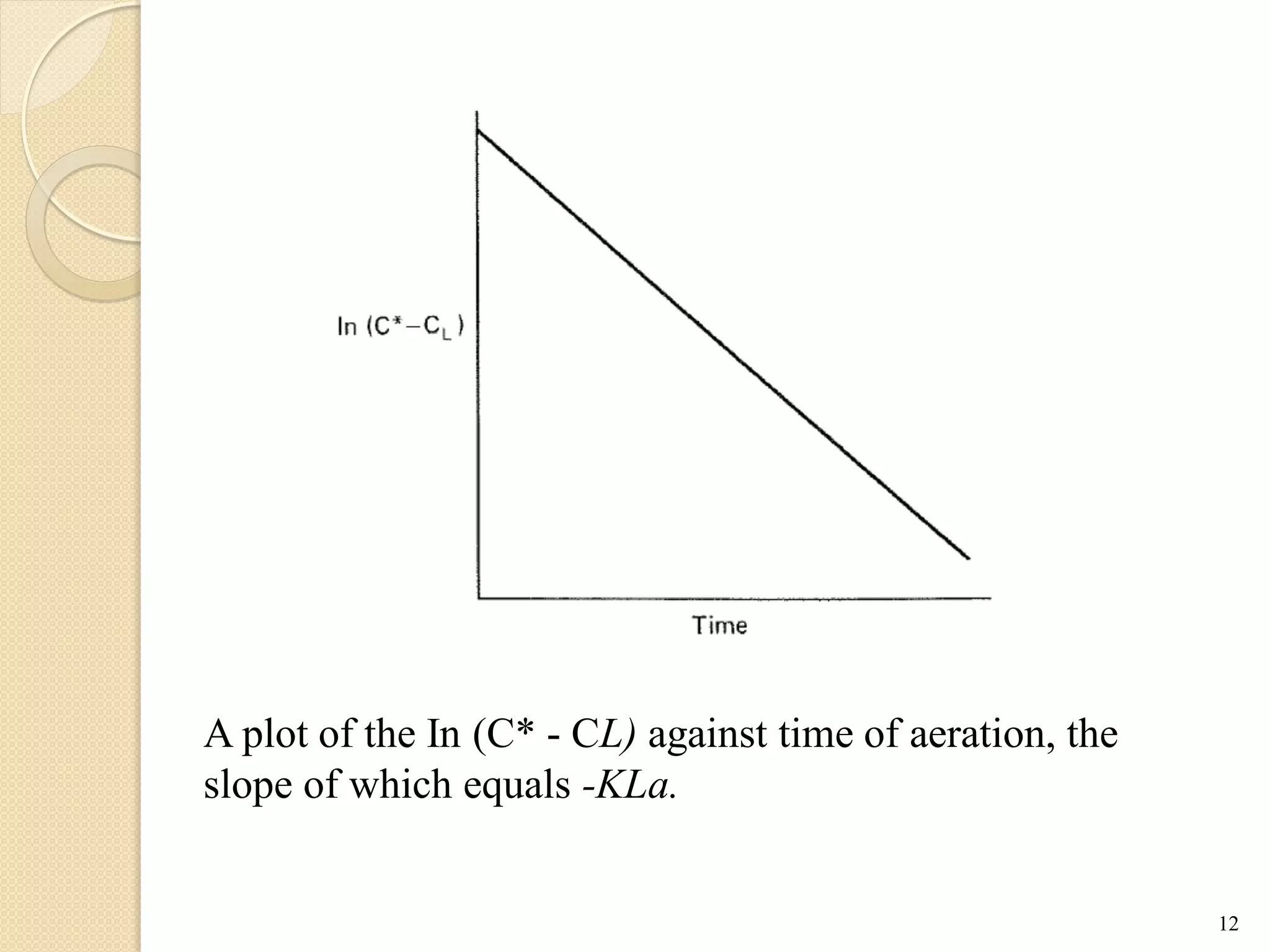
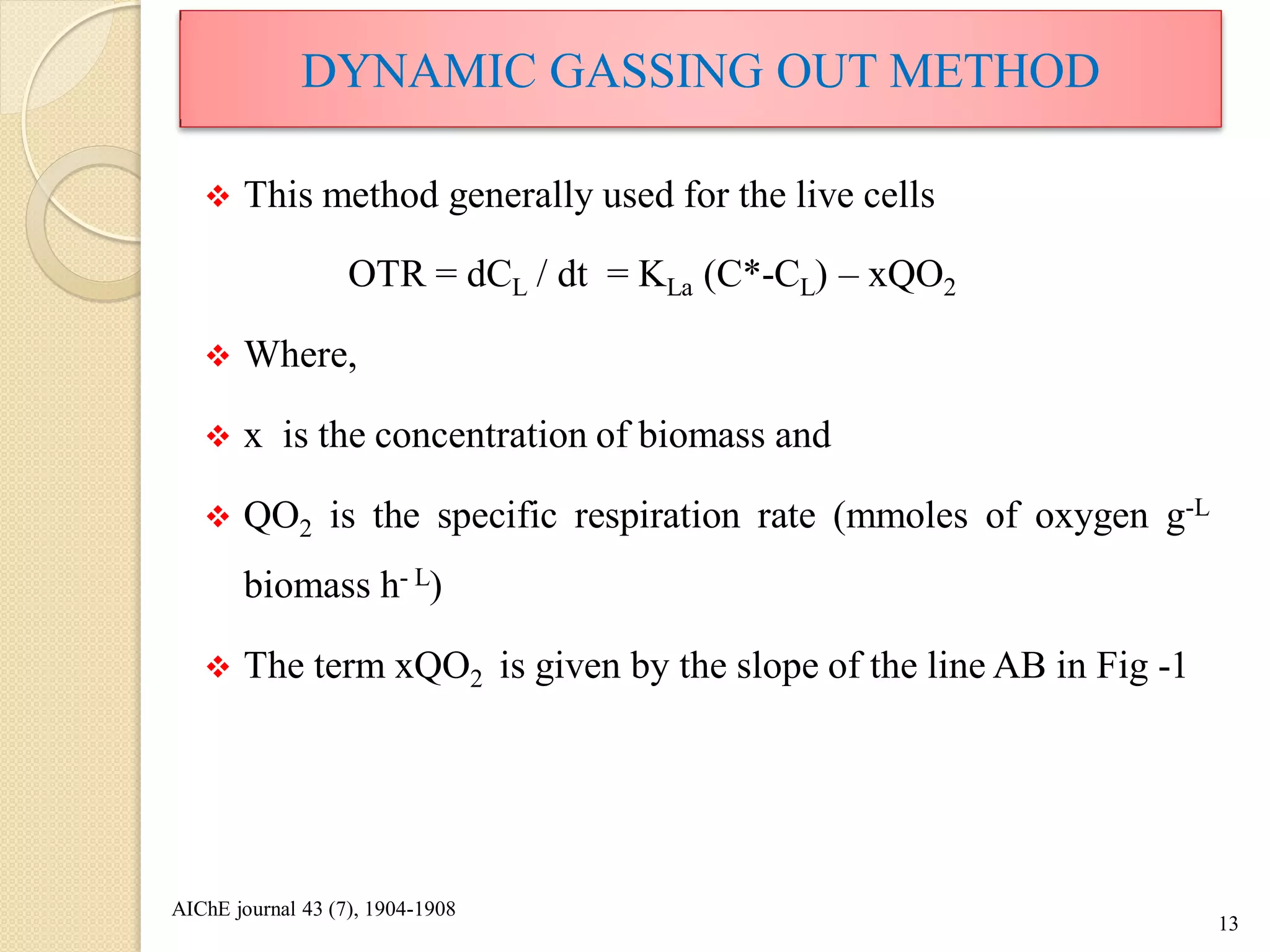
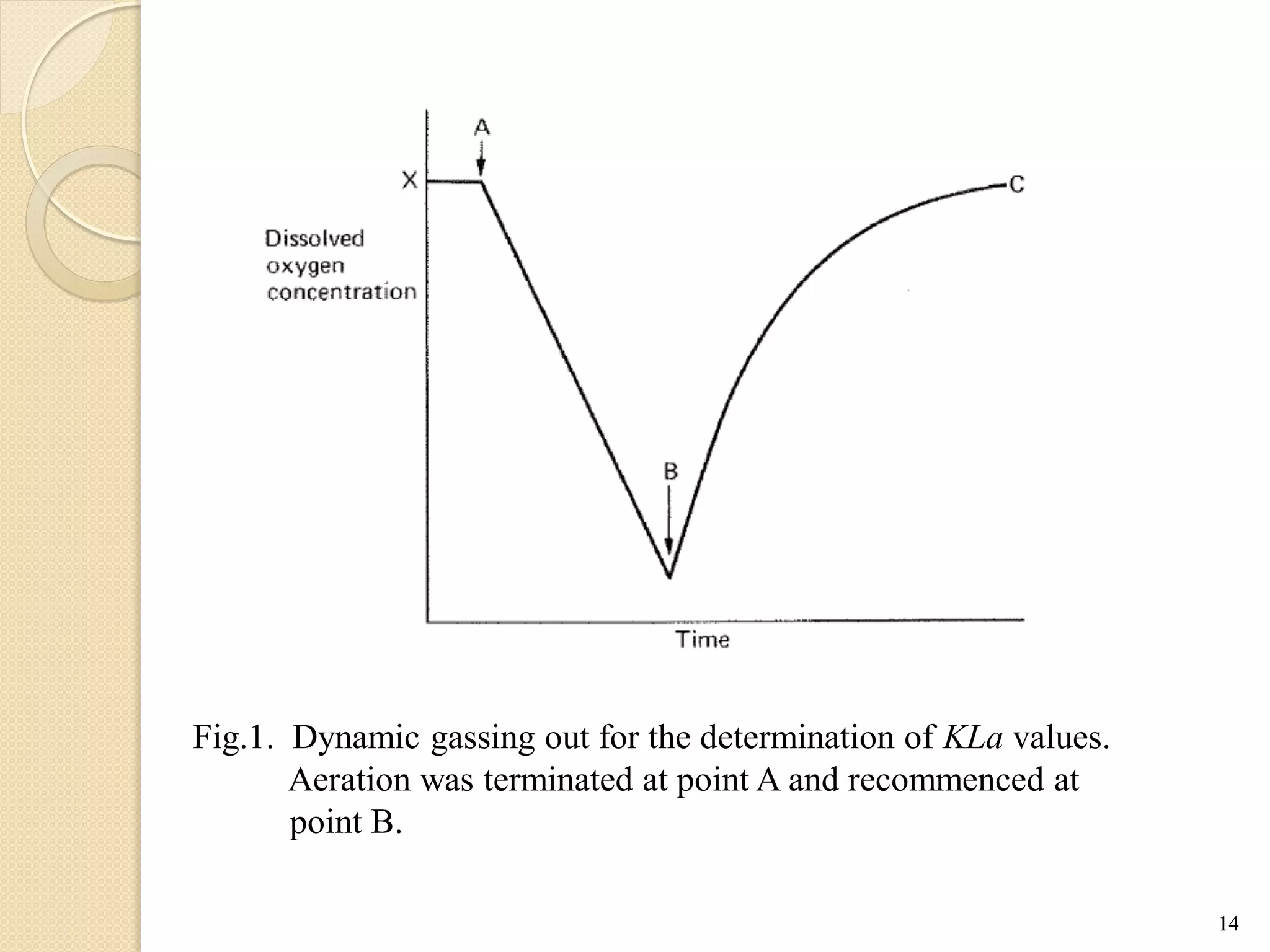
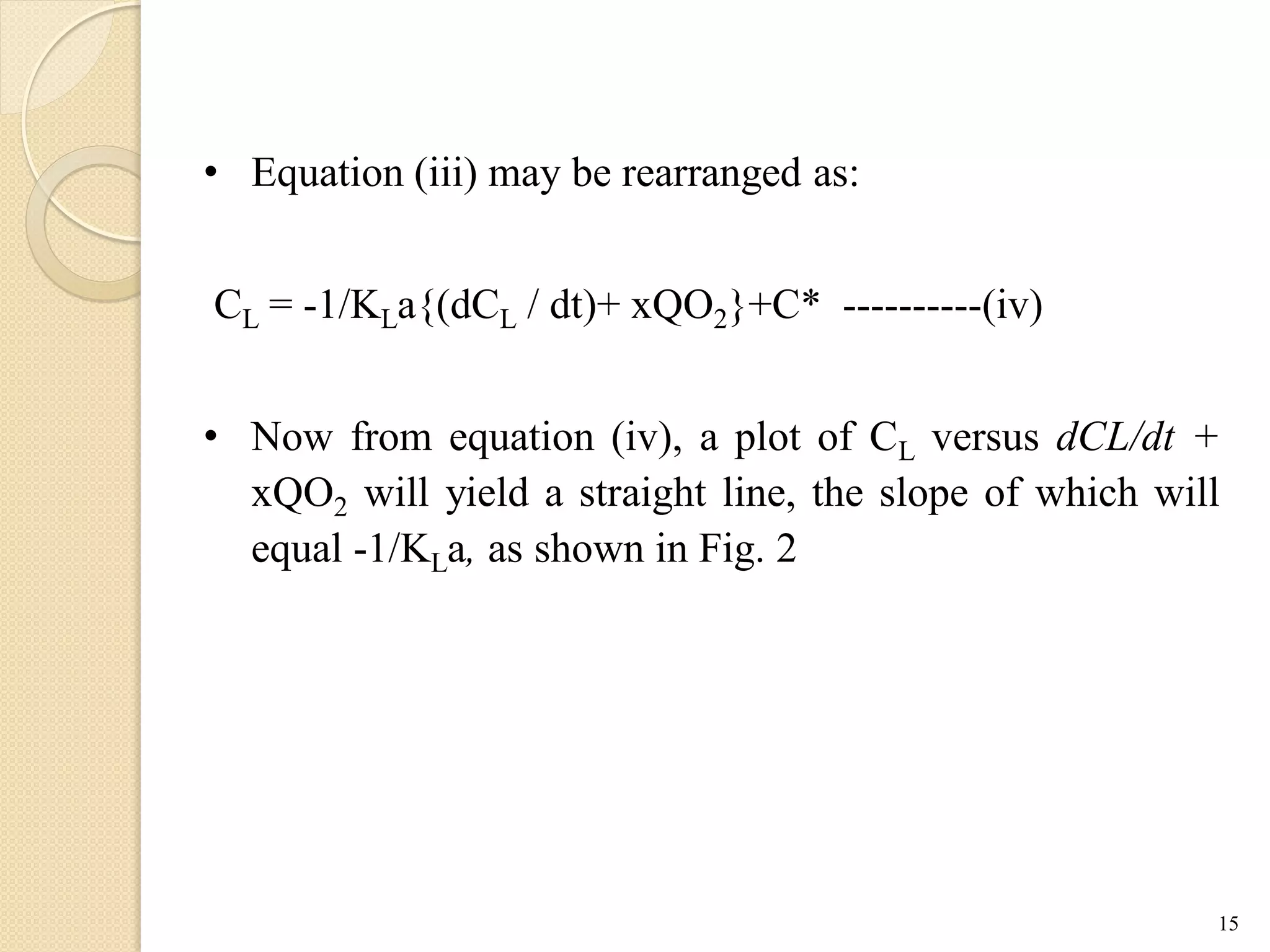
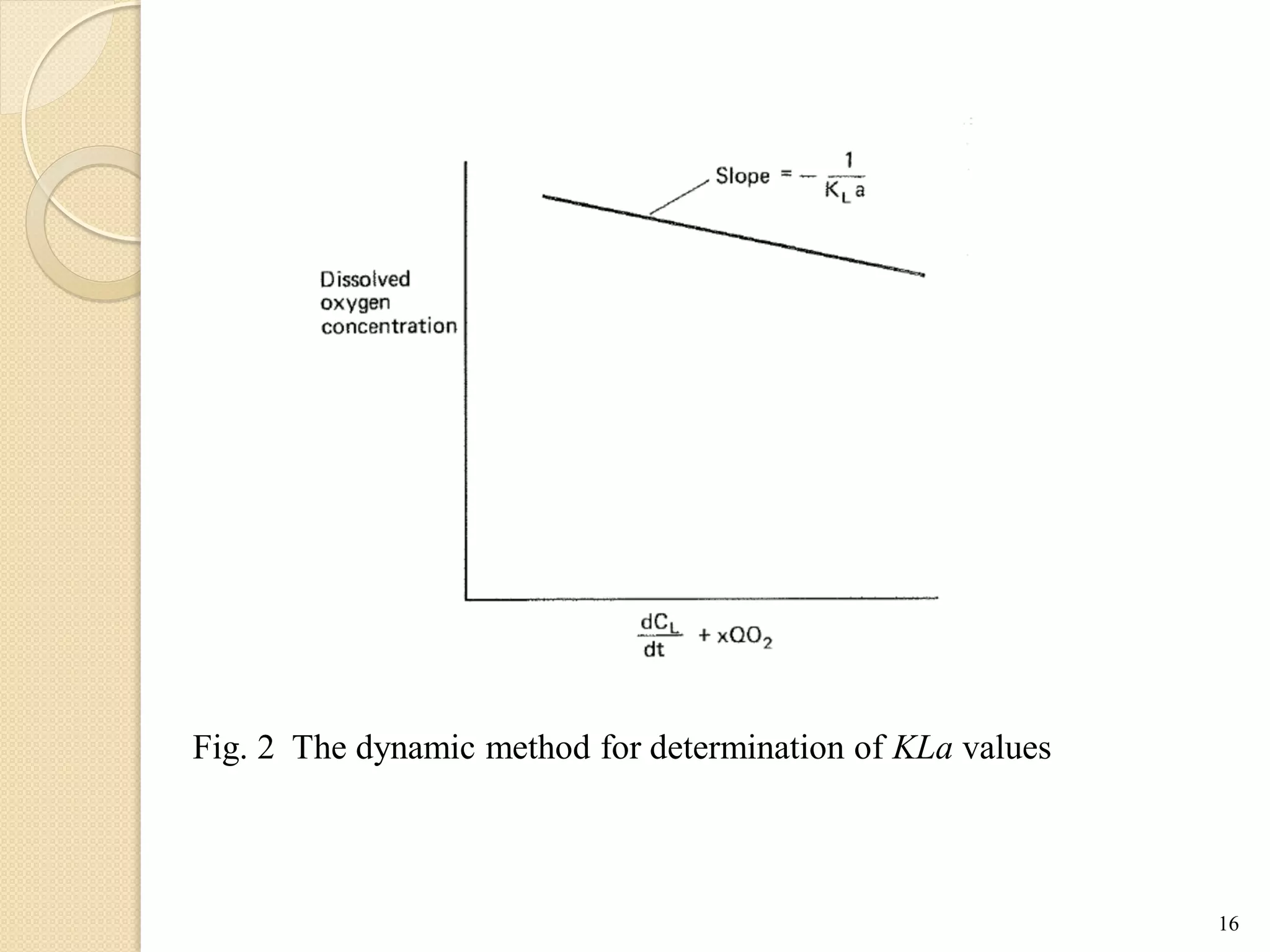
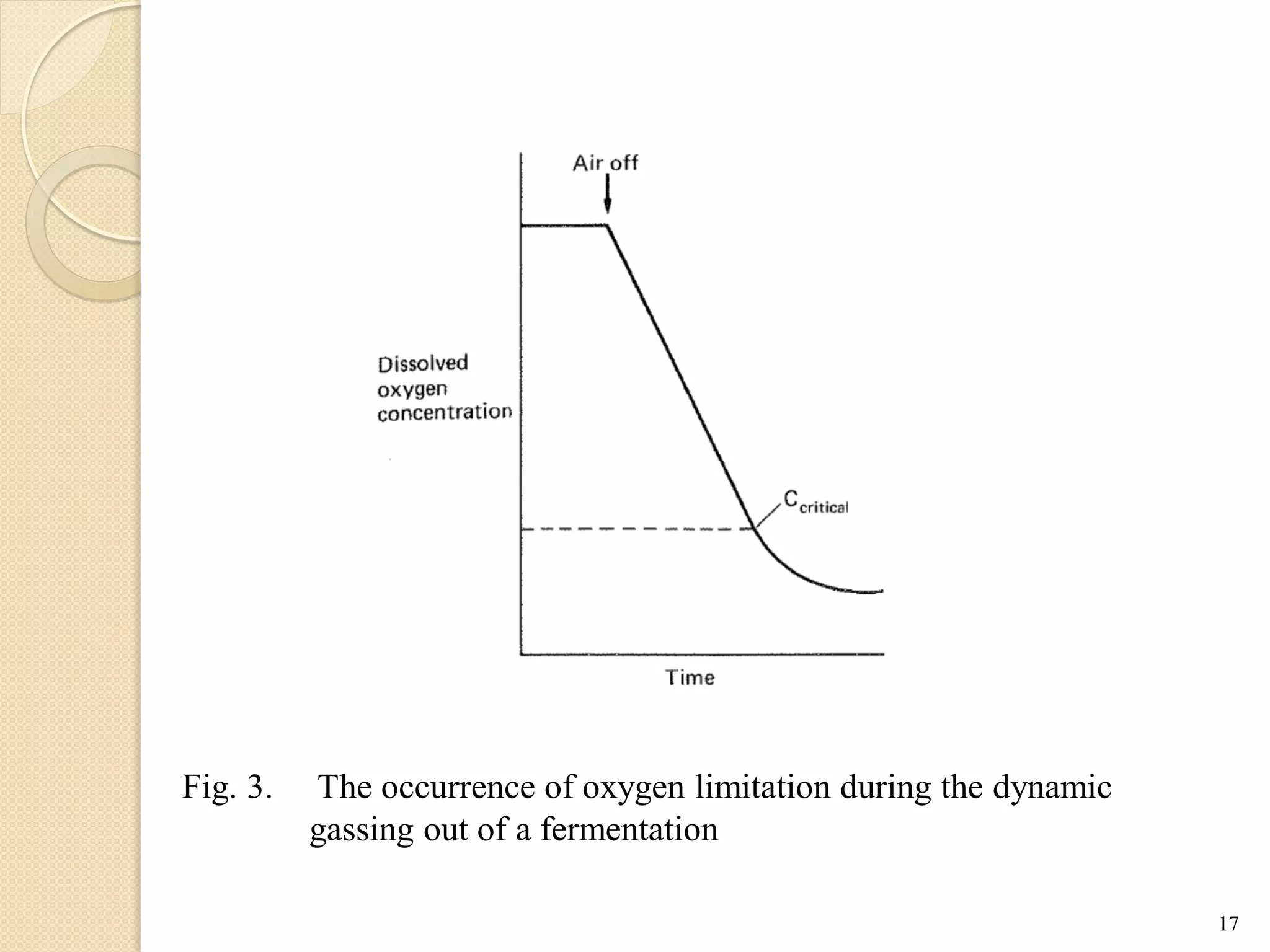
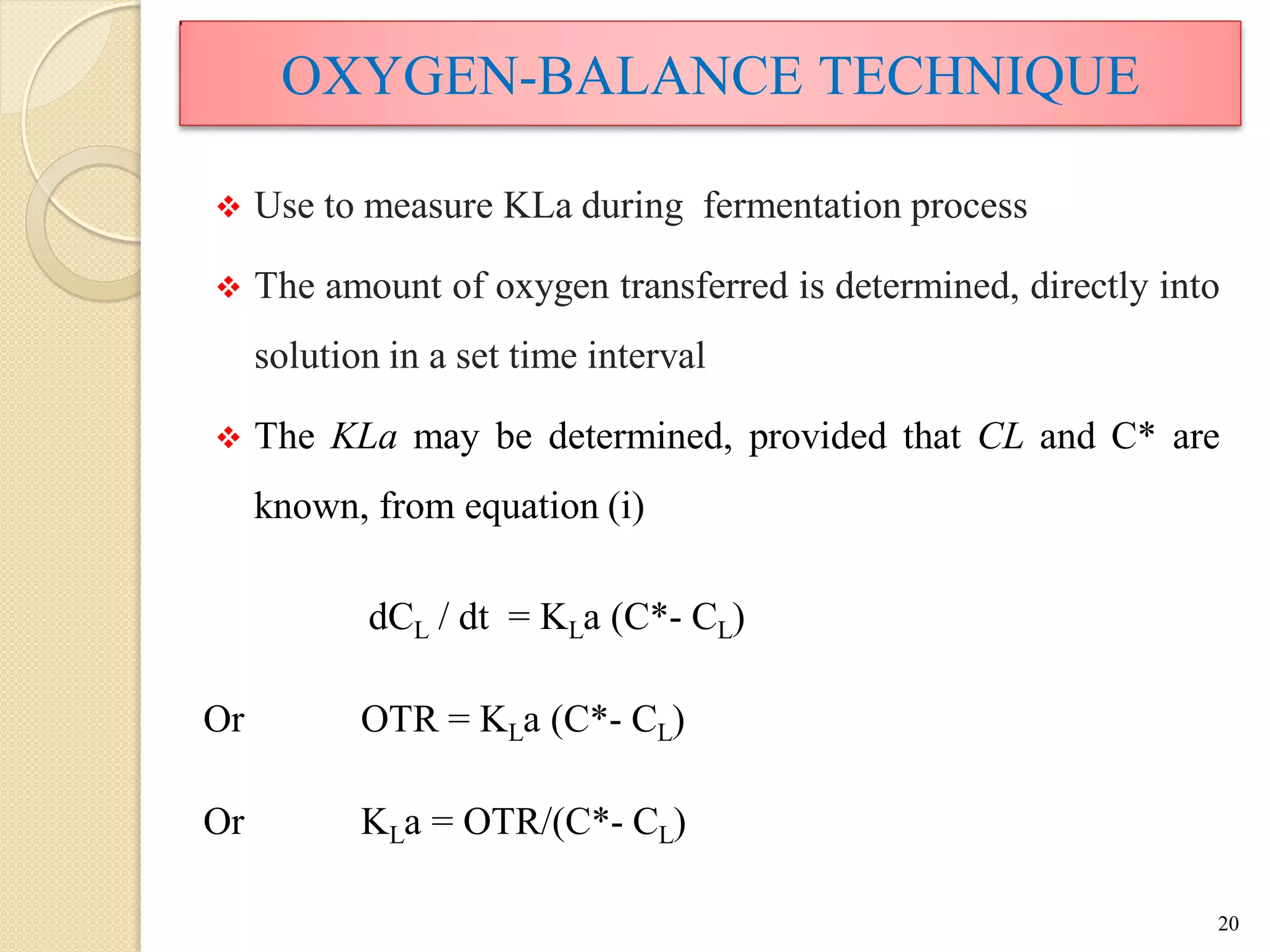
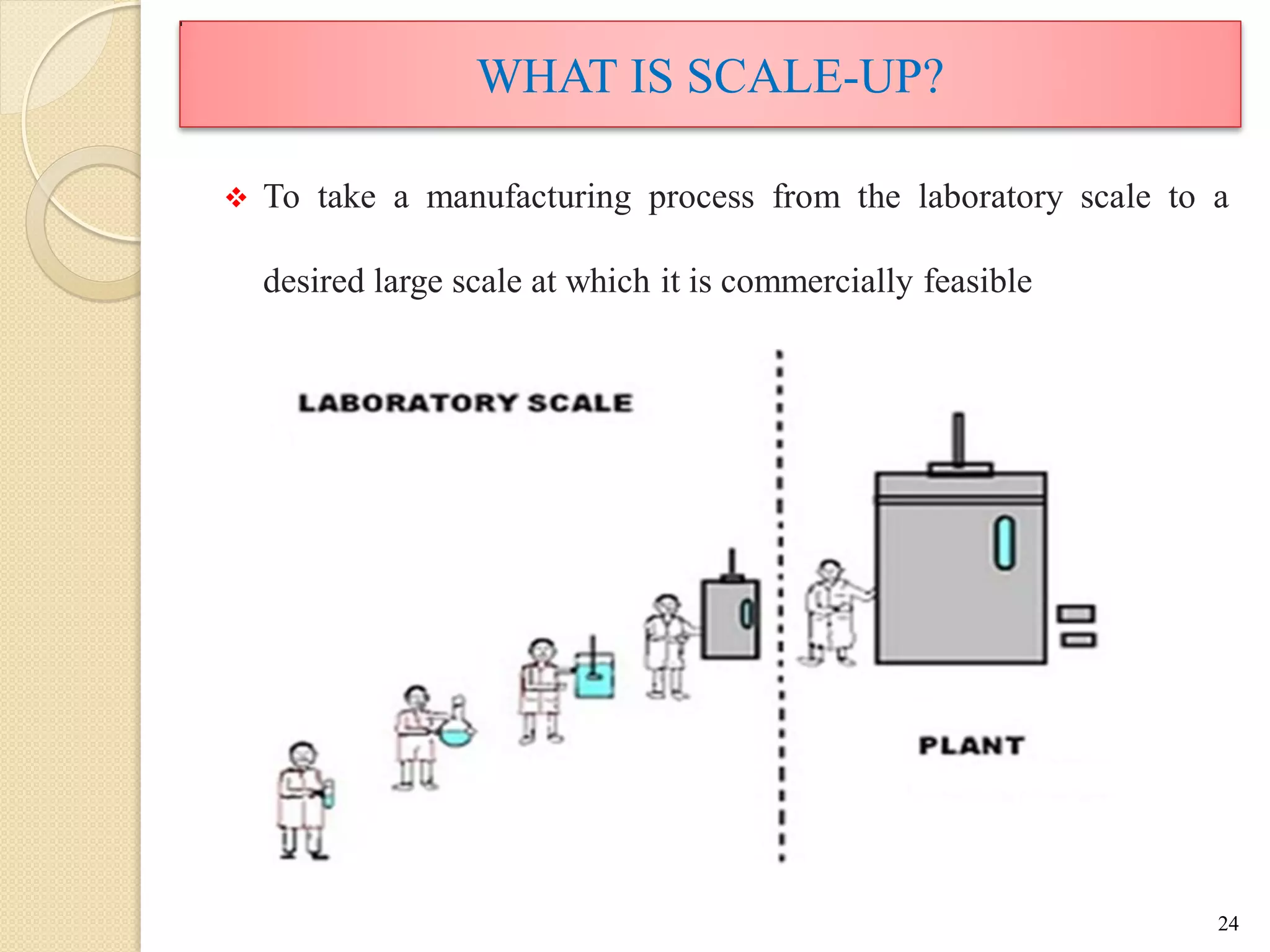
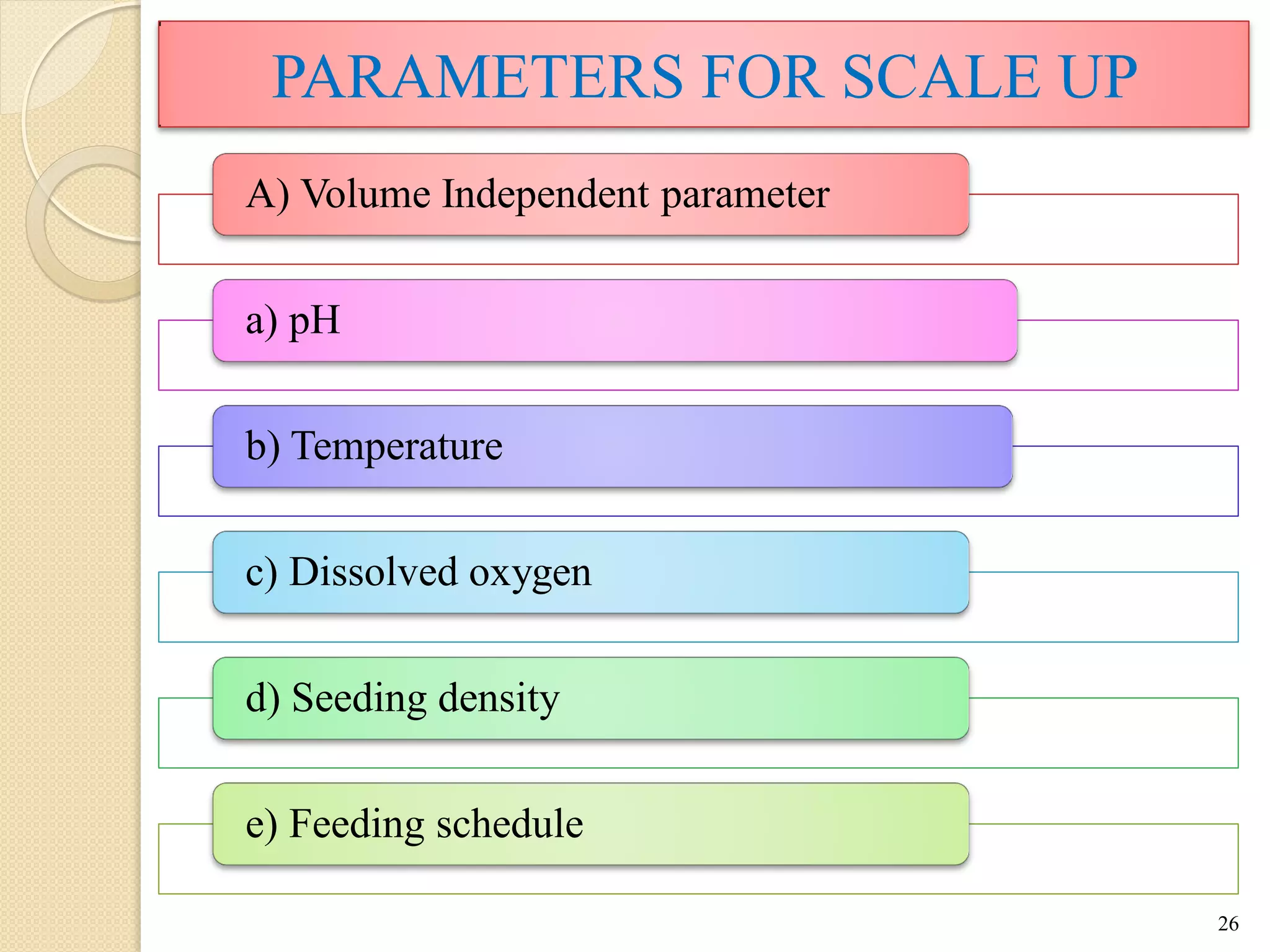
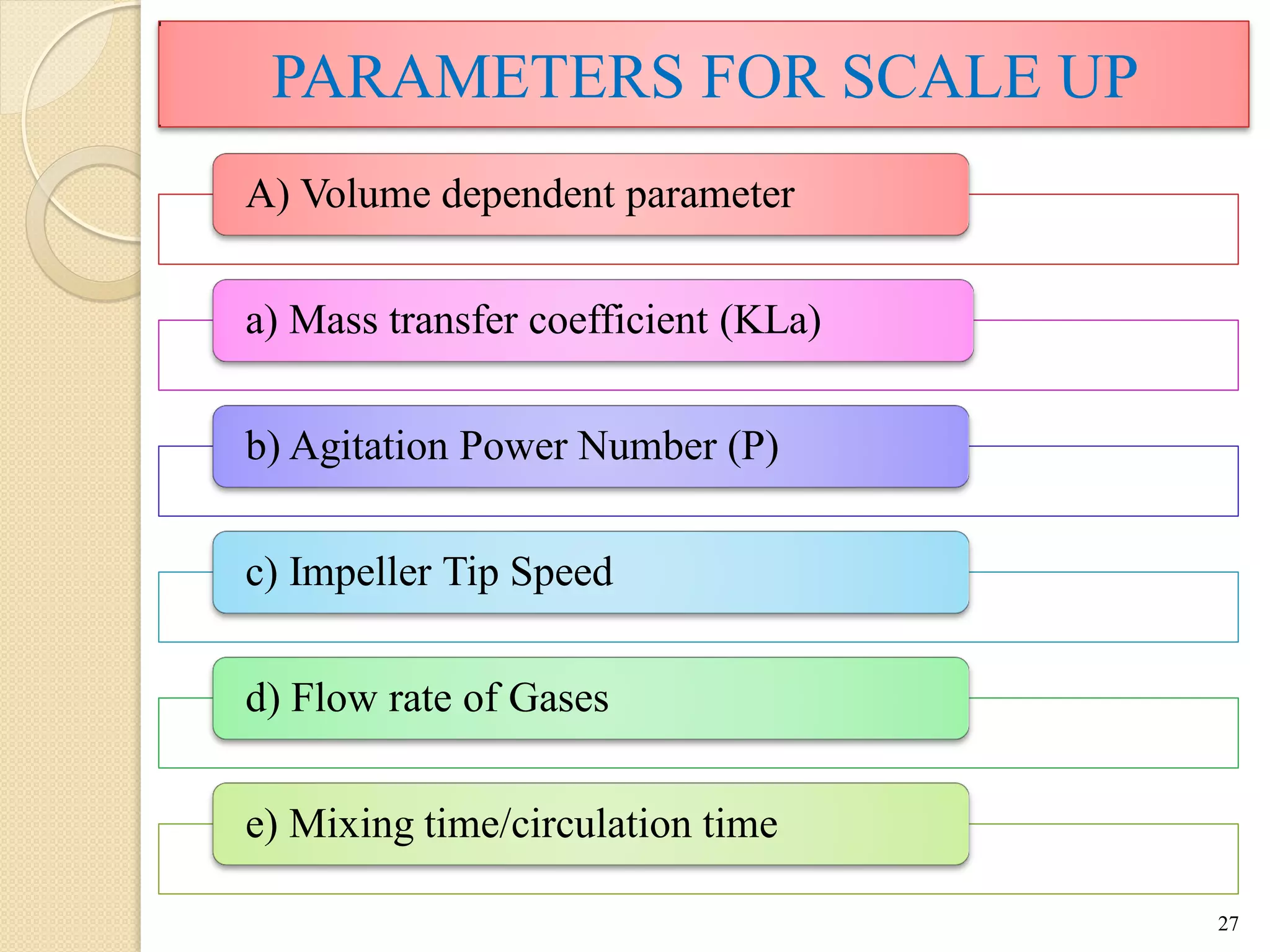
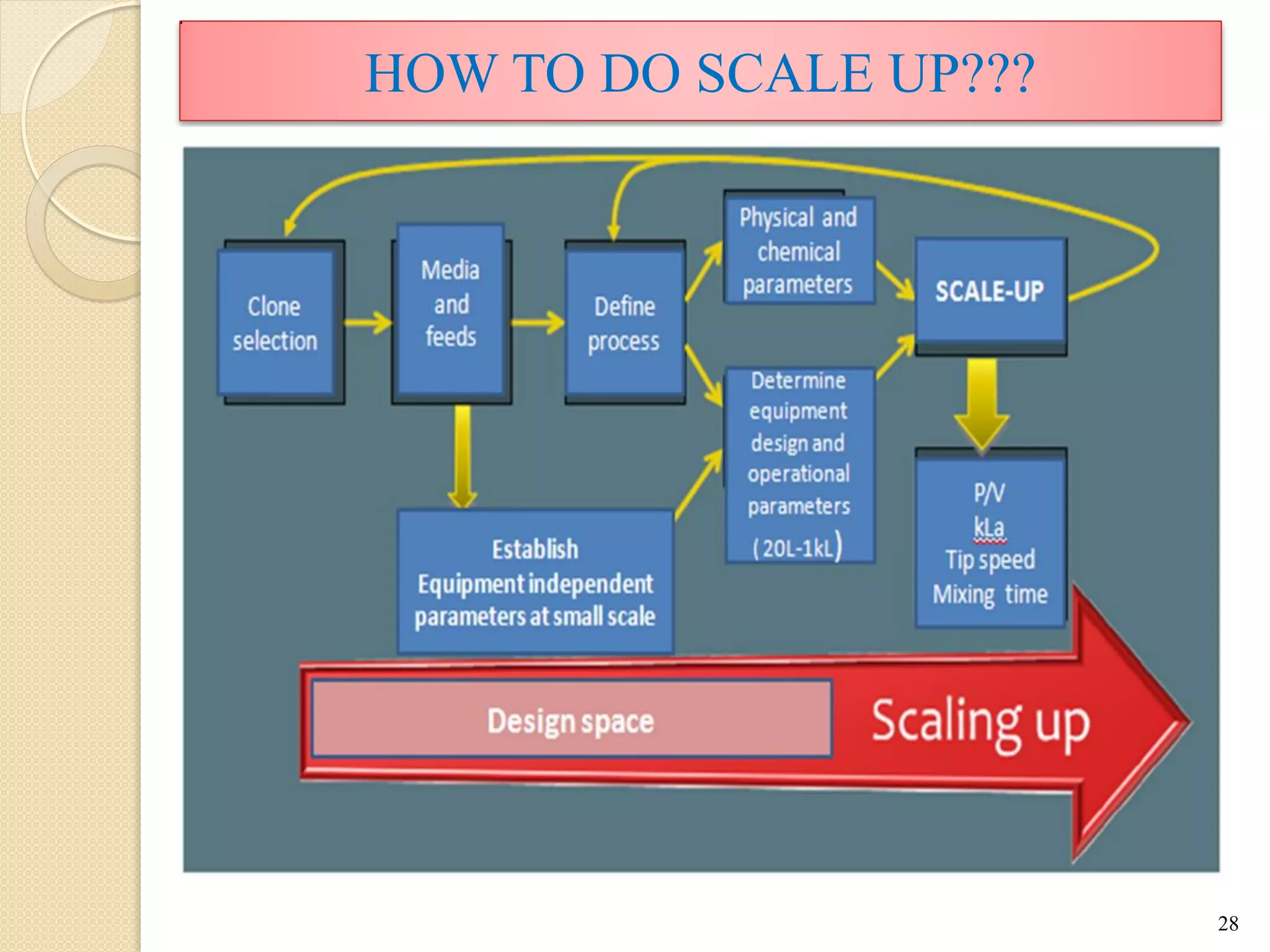
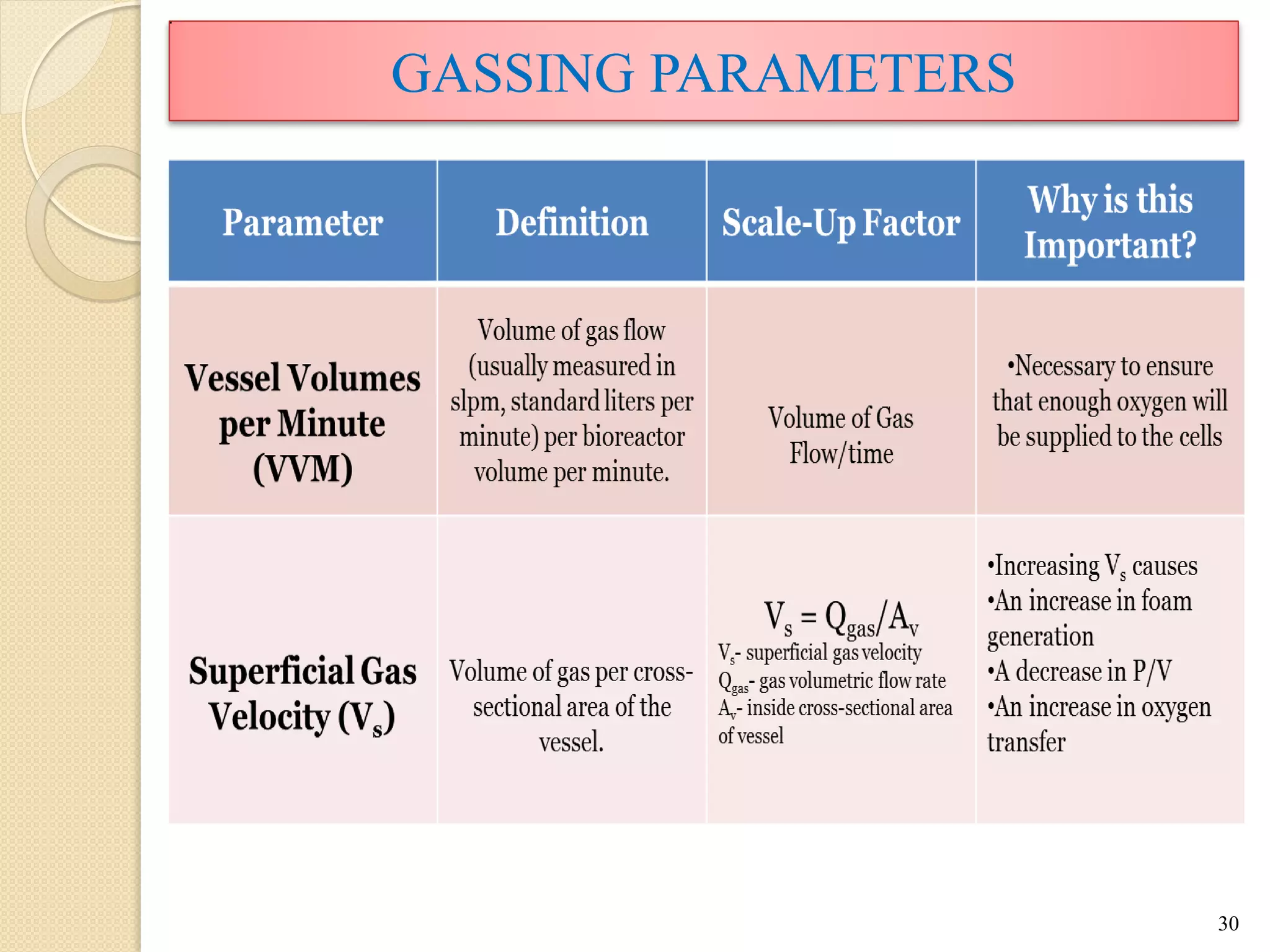
The document discusses measurement of the volumetric mass transfer coefficient (KLa), which indicates the rate of oxygen transfer in a bioreactor. It describes various methods to determine KLa values, including chemical and physical techniques like the sodium sulphite oxidation method. The document also covers factors that affect KLa, and how KLa values are used to scale bioreactors from laboratory to production scale.