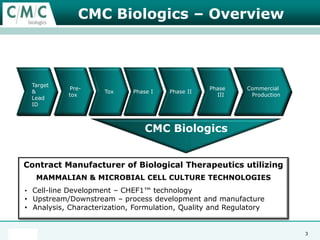
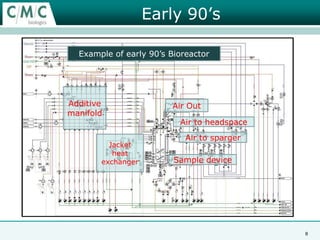
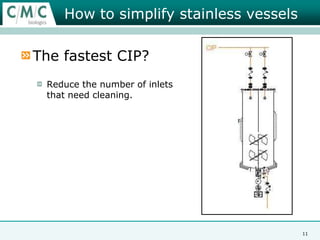
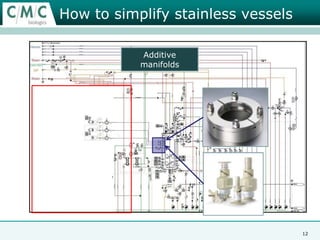
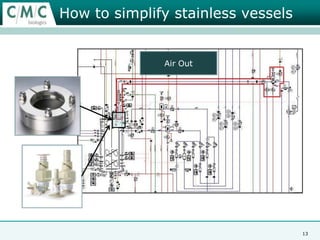
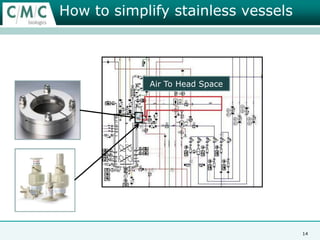
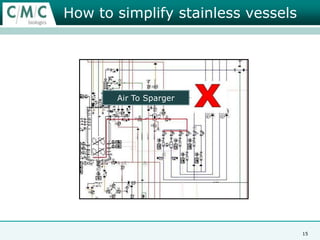
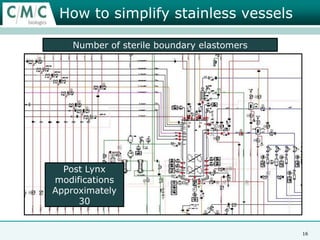
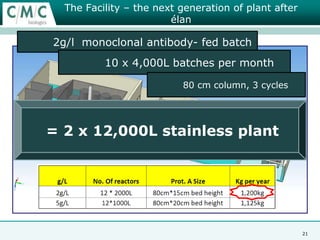
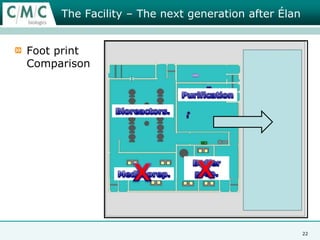
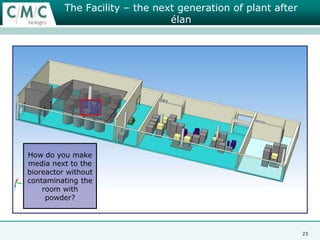
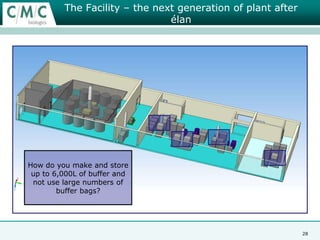
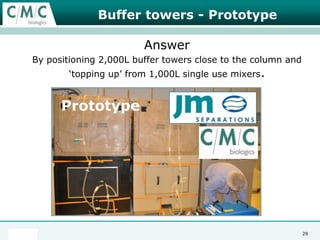
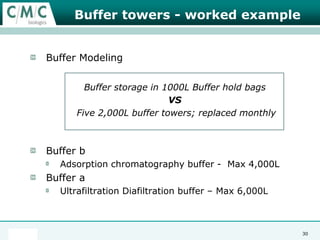
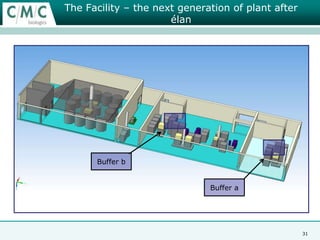
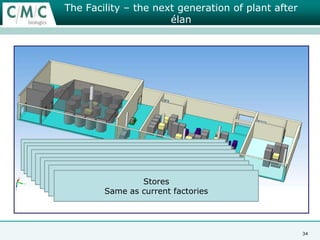
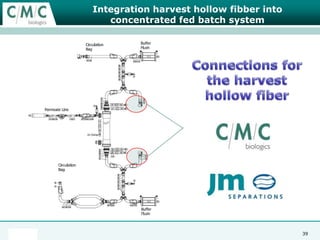
This document discusses the development of large-scale mammalian cell culture facilities from the 1980s to present day. Early facilities in the 1980s utilized simple semi-manual systems with basic automation and validation. Facilities evolved in the 1990s to reduce complexity and costs through simplifying stainless steel bioreactors and adopting single-use disposables. Present day facilities since 2008 widely accept large-scale disposable bioreactors up to 2000L in volume. The next generation of facilities aims to further reduce footprint and costs through integrated single-use systems for media preparation, buffer storage, and harvesting.