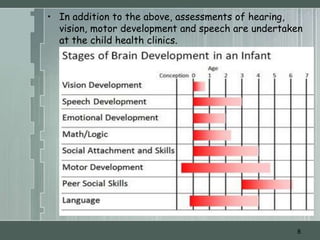
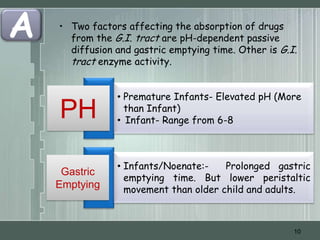
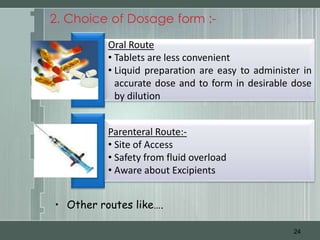
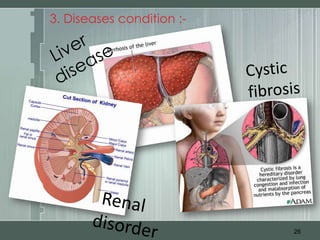
The document discusses important considerations for pediatric drug handling. It covers pharmacokinetic parameters like absorption, distribution, metabolism, and excretion that differ in children compared to adults due to developmental changes. The document emphasizes the need for pediatric clinical trials to determine appropriate drug dosages, formulations, and administration methods for children of different ages. Monitoring growth, vital signs, and laboratory parameters is also important for safe drug therapy in children.