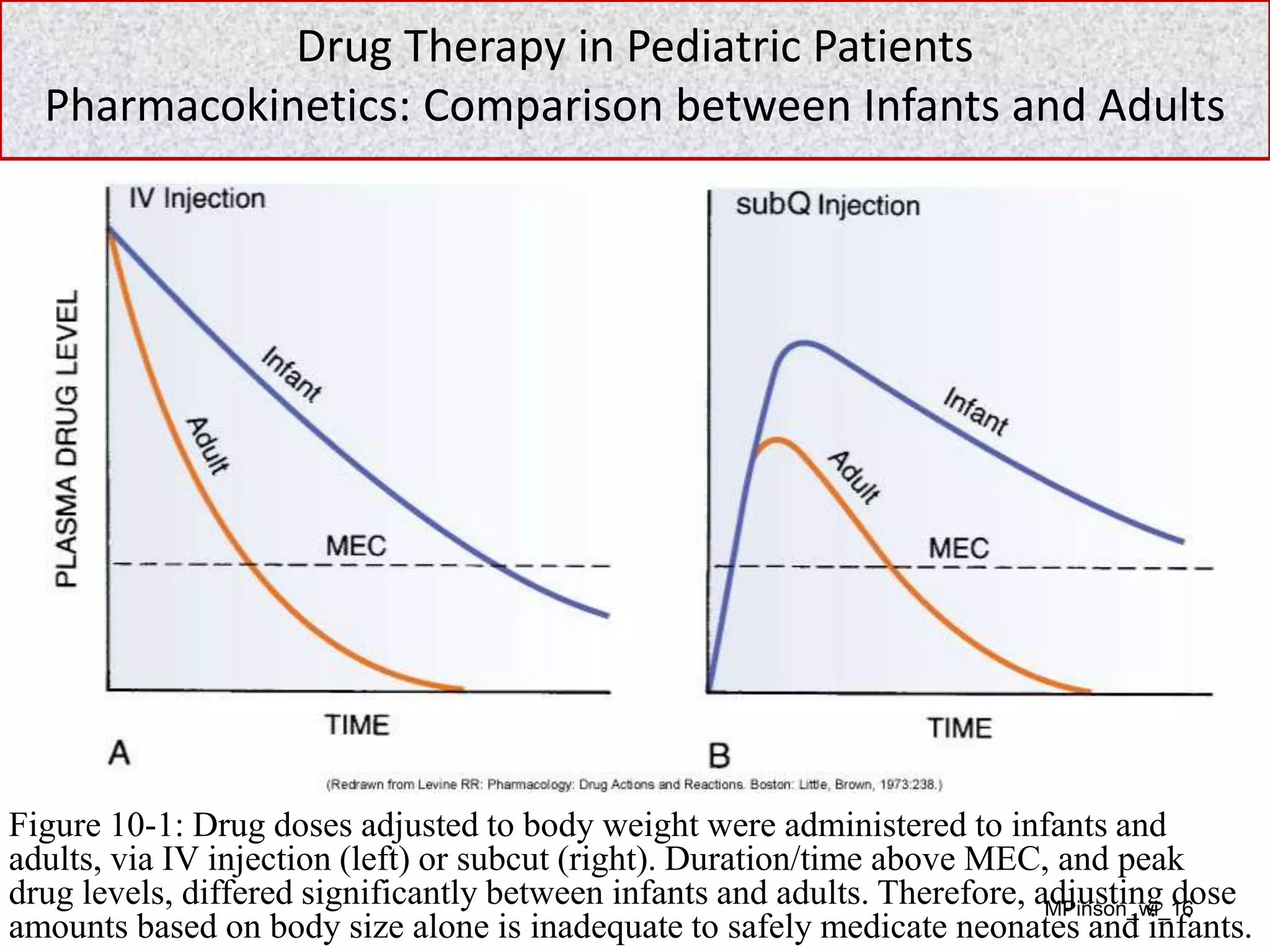
- Drug therapy in pediatric patients presents unique challenges due to physiological differences compared to adults that influence pharmacokinetics. Organs such as the liver and kidneys are immature at birth and do not reach adult functionality until approximately 1 year of age. This results in altered absorption, distribution, metabolism, and excretion of drugs in neonates and infants.
- Due to organ immaturity, neonates and infants experience more intense and prolonged responses to drugs. They are at higher risk for adverse effects from drugs cleared primarily by the liver or kidneys. Careful monitoring is needed when dosing pediatric patients.
- Initial pediatric doses are approximations, often based on body surface area calculations. Frequent assessment and potential dose adjustments are