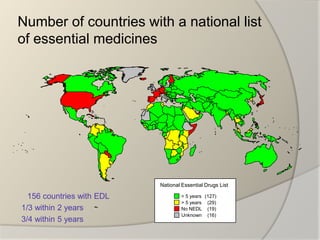
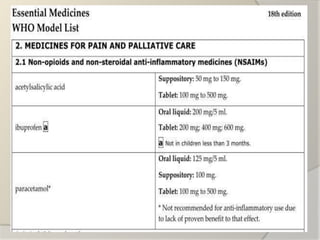
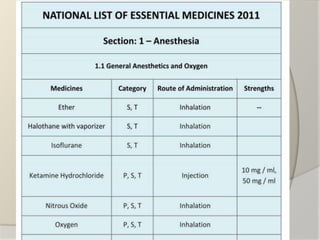
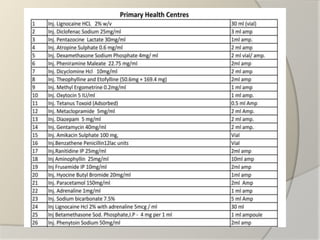
The document discusses the concept of essential medicines and rational use of drugs. It defines essential medicines as those that meet the priority health care needs of the population. The WHO publishes a Model List of Essential Medicines every two years to guide countries in developing their own national lists. Educational, managerial, economic and regulatory strategies can be used to promote rational drug use and selection of cost-effective treatments. Pharmacists can play a role through drug selection, inventory control, patient education, and pharmaceutical care.