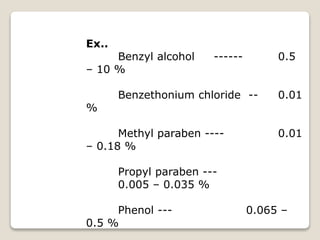
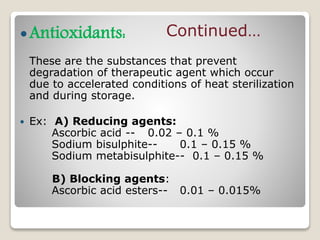
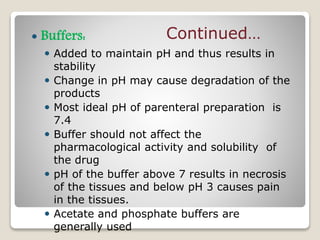
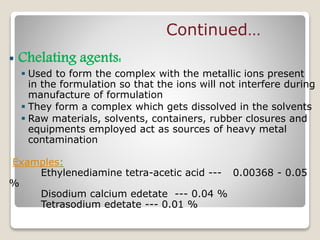
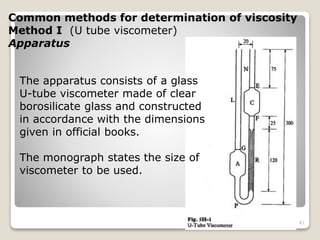
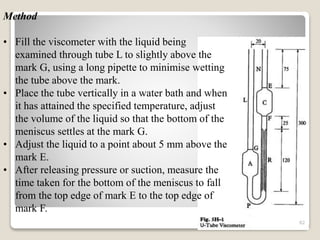
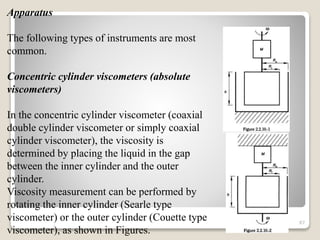
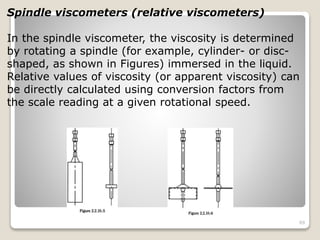
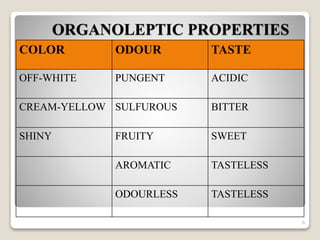
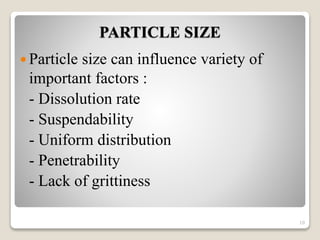
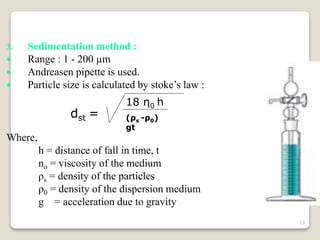
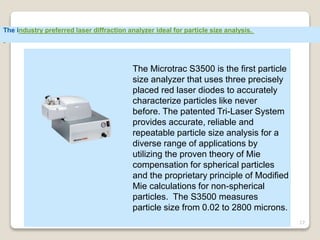
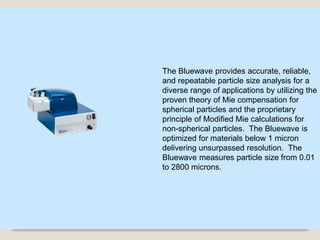
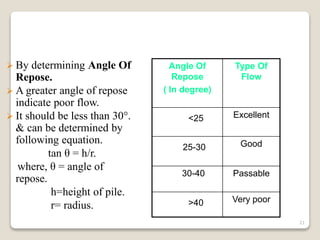
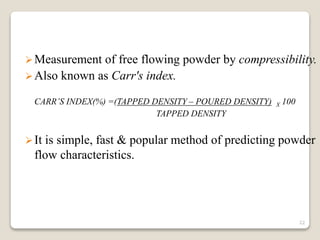
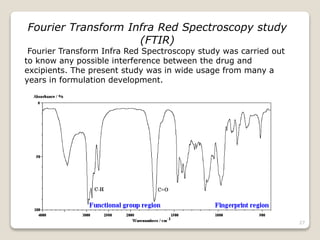
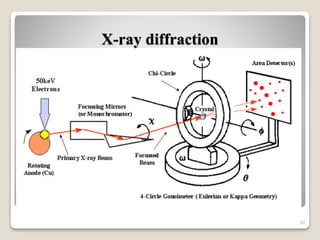
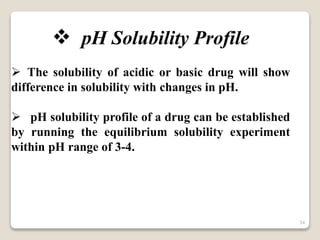
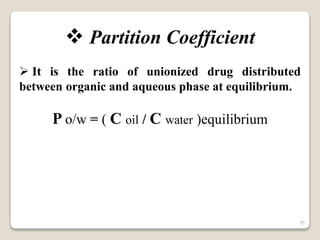
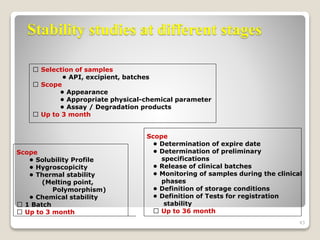
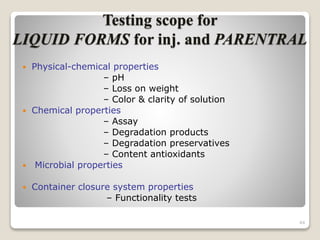
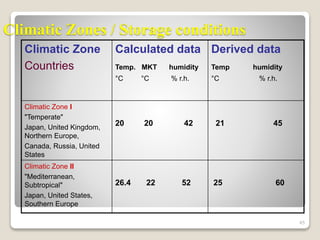
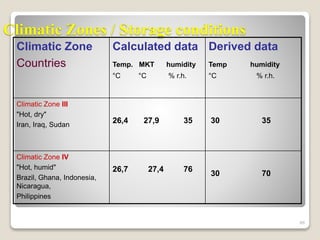
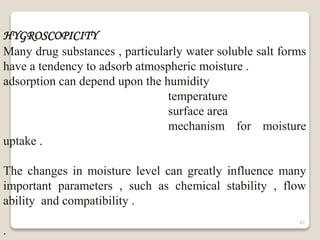
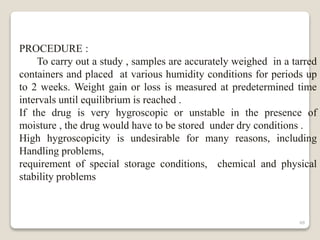
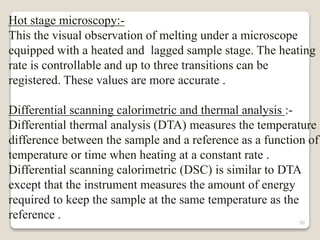
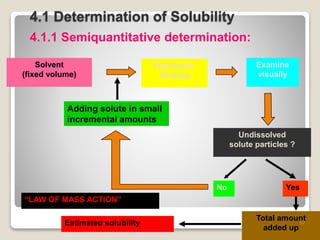
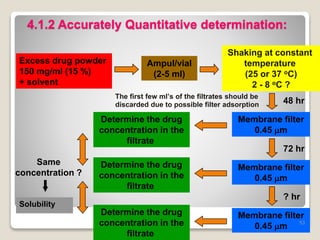
This document provides an overview of preformulation studies for intramuscular injections. It discusses various parameters studied in preformulation including organoleptic properties, particle size, shape and distribution, powder flow properties, FTIR spectroscopy, DSC, X-ray diffraction, solubility, partition coefficient, and the effect of temperature on solubility. It also describes stability studies conducted at different stages including stress testing, accelerated and long-term testing to determine shelf life and storage conditions. The testing scope for liquid injectables includes parameters like pH, clarity, assay, degradation products, and functionality of container closure systems. Finally, it discusses climatic zones for stability testing and the hygroscopic nature of some drug substances.

![Intramuscular (also IM or im) injection is
Intramuscular injection
the injection of a substance directly into a muscle.
In medicine, it is one of several alternative methods for the
administration of medications (see route of administration).
It is used for particular forms of medication that are
administered in small volumes
Intramuscular injection
Depending on the chemical properties of the drug, the
medication may either be absorbed fairly quickly or more
gradually. Muscles have larger and more blood vessels than
subcutaneous tissue and injections here usually have faster
rates of absorption than subcutaneous
injections or intradermal injections.[1] Depending on the
injection site, an administration is limited to between 2 and 5
milliliters of fluid.
2](https://image.slidesharecdn.com/198814ed-c8b9-4c68-a523-5c170bea9c07-160305062108/85/rina80-2-320.jpg)














![ Ionization constant (pKa)
Can be calculated by Henderson Hasselbach
equation-
For acidic drugs….pH= pKa+ log [ionized drug]
[unionized drug]
For basic drugs….pH= pKa+ log[unionized drug]
[ionized drug]
33](https://image.slidesharecdn.com/198814ed-c8b9-4c68-a523-5c170bea9c07-160305062108/85/rina80-33-320.jpg)









![ Poorly-soluble weakly-acidic drugs:
pH = pKa + log [(St - So)/So] (2)
Poorly-soluble weakly-basic drugs:
pH = pKa + log [So/(St - So)] (3)
where
So = solubility of unionized free acid or base
St = total solubility (unionized + ionized)
56](https://image.slidesharecdn.com/198814ed-c8b9-4c68-a523-5c170bea9c07-160305062108/85/rina80-56-320.jpg)