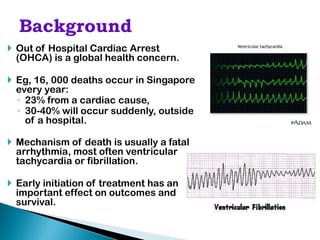
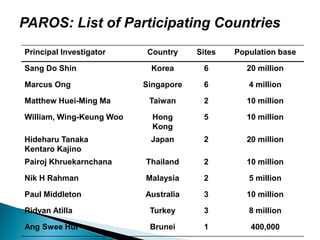
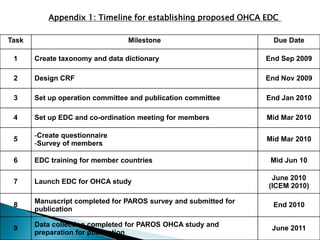
This document proposes establishing the Pan-Asian Resuscitation Outcomes Study (PAROS) to track out-of-hospital cardiac arrest across Asia. It would collect data on incidence, outcomes, and emergency medical systems from multiple countries to allow for comparisons and identify best practices. The study would be modeled after the Cardiac Arrest and Resuscitation Epidemiology study in Singapore and involve over 13,000 cases collected electronically using common definitions and procedures. Participating countries would study populations totaling over 100 million people. The goal is to understand and improve resuscitation systems to reduce deaths from out-of-hospital cardiac arrest across the region.