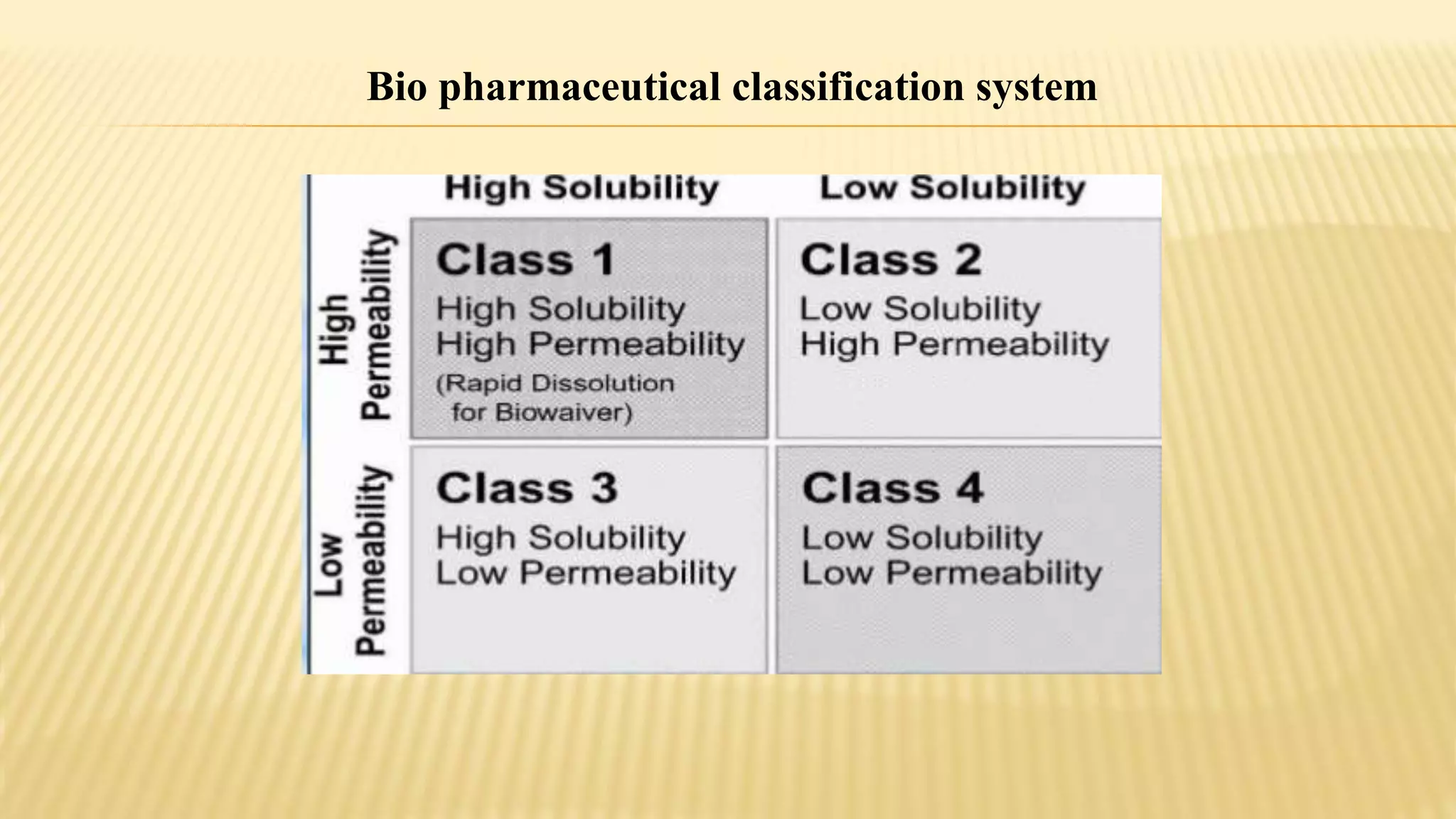
This document discusses bioequivalence studies. It defines bioequivalence as when two drug products reach systemic circulation to the same relative extent, with their plasma concentration-time profiles being identical without statistically significant differences. It describes the analytical methods, pharmacokinetic evaluation, and statistical evaluation used in bioequivalence studies. It also discusses study designs such as parallel designs, crossover designs, and fasting versus fed conditions that can be used in bioequivalence studies.


![Pharmacokinetic evaluation of the data:
• single dose study: AUC0-t,AUC0-∞, Tmax, Cmax, Cmin and percent
fluctuation[100*(Cmax-Cmin)/Cmin].
Statistical evaluation of the data:
• No statistical difference between the bioavailability of the test product and
the reference product.
• Bell-shaped curve
• Log values resembles more closely a normal distribution
Analysis of variance(ANOVA):
• No significance difference
• AUC0-∞, Tmax&Cmax](https://image.slidesharecdn.com/abppk-190827170158/75/bio-equivalence-studies-3-2048.jpg)


























![ Evaluation of data:
Statistical Evaluation
Primary concern of bioequivalence is to limit Consumer’s &
Manufacturer’s risk
Cmax & AUC analysed using ANOVA
Tmax analysed by non-parametric methods
Use natural log transformation of Cmax and AUC
Calculate Geometric means of Cmax of Test [Cmax’t]
Calculate Geometric means of Cmax of Reference [Cmax’r]
Calculate Geometric Mean Ratio = [Cmax’t] / [Cmax’r]
Calculate 90% confidence interval for this GMR for Cmax
Similarly calculate GMR for AUC](https://image.slidesharecdn.com/abppk-190827170158/75/bio-equivalence-studies-30-2048.jpg)