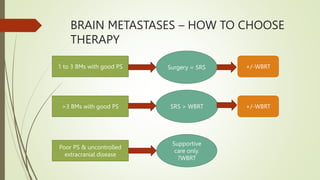
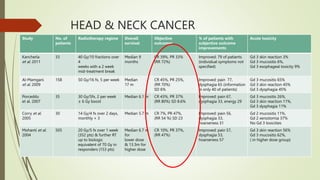
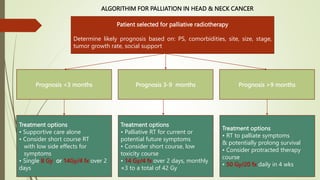
This document discusses palliative radiotherapy. It begins with a brief history of palliative radiotherapy and a definition of palliative care. It then discusses the differences between curative and palliative radiotherapy in terms of aims, doses, fractions, and toxicities. Clinical indications for palliative radiotherapy in bone metastases, brain metastases, and malignant spinal cord compression are reviewed. Modern technologies and caveats are also mentioned. Studies comparing different fractionation schedules and treatment approaches are summarized. Patient selection factors and how to choose appropriate therapy based on prognosis are outlined.

![“The final causes, then, of compassion are to prevent
and to relieve misery.”
Joseph Butler [1692–1752]](https://image.slidesharecdn.com/pallrt-230519234310-2a401ed2/85/Pall-RT-pptx-2-320.jpg)


![HISTORY
X-rays discovered by Wilhelm Roentgen in Nov 1895.
Emille Grubbe claimed to have successfully palliated a patient of Ca Breast with X-
rays in Jan 1896.
Lenz & Freid in Ann Surg, 1931, evaluated and found moderate doses of RT
effective in palliation of brain, spine & bone metastases.1
A review in the BMJ in 1957 reported that a dose of 3000 rad was sufficient for
palliation. Balancing benefit with harm from palliative radiotherapy was now the
task of the radiotherapist. 2
Chu et al in 1961 described a symptomatic response in 77.8% (123 of 158) patients
of brain metastases treated with 3000 rad of WBRT. 3
1. Lenz M, Freid J. Metastases to the skeleton, brain and spinal cord from cancer of the breast and the effect of radiotherapy. Ann Surg [Internet] 1931; 93: 278–293.
2. Palliative radiotherapy. BMJ 1957; 2: 455–456.
3. Chu FCH, Hilaris B, Chu FC, et al. Value of radiation therapy in the management of intracranial metastases. Cancer [Internet] 1961.](https://image.slidesharecdn.com/pallrt-230519234310-2a401ed2/85/Pall-RT-pptx-5-320.jpg)















![ RT can effectively improve QoL by relieving pain & compressive symptoms and
recovering or preserving motor & bladder function when initiated within time
No difference in single vs multiple fx schedules of RT in recovery of ambulation
Surgical decompression along with RT gives better improvement of neurodeficit but
careful selection is imp.1
Survival related to primary but pts of MSCC with no motor dysfunction live longer
64% of recurrence occur within 2 vertebral bodies of initial site2
Re-irradiation can be considered within dose constraints (BED -120Gy)
Indications of SBRT include recurrence after RT or oligometastic dx
PALL RT IN MALIGNANT SPINAL CORD COMPRESSION
1 - Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet
2005;366:643-8. 10.1016/S0140-6736(05)66954-1 [PubMed: 16112300]
2 - Maranzano E, Trippa F, Chirico L, et al. Management of metastatic spinal cord compression. Tumori 2003; 89: 469–475.](https://image.slidesharecdn.com/pallrt-230519234310-2a401ed2/85/Pall-RT-pptx-22-320.jpg)




![RADIOTHERAPY FOR PALLIATION IN
GYNAECOLOGICAL MALIGNANCIES
Author & year of
publication
Radiation dose
(Gy) per
fraction/
No.
of fractions
No. of
patients
>50% improvement in symptoms > 50% tumor
response
Complications
Bleeding Pain Obstruction
No. (%) No. (%) No. (%)
Halle et al.
1986 [6]
10 Gy/1-3 42 27/30 (90)) 4/9 (44 15/28 (54) 5/42 (12%)
2 also had
uncontrolled
pelvic tumor
Spanos et al.
1989 [8]
RTOG 8502
3.7 Gy/4 in 2
days, 3 such
cycles
136 71/73 (97) 44/65 (68) 14/16 (88) 41/136 (30)
40/92 (43)
for pts receiving all 3
fractions
10/87 (12%)
Gr-3 late
morbidity
Mishra et al.
2005 [9]
10 Gy/1
10 Gy/2
10 Gy/3
100
61
33
(31)
(100)
(3)
(41)
(17)
24/70 (34)
28/45 (62)
25/33 (75)
10 pts (10%) had
Gd 3–4 late
morbidity
van Lonkhuijzen
et al. 2011,SR
10Gy/1 x 3 or
2-4 Gy
fractions
476 in 8 studies 45 to 100 % 31 to 100% 15 to 100% Not reported
consistently](https://image.slidesharecdn.com/pallrt-230519234310-2a401ed2/85/Pall-RT-pptx-29-320.jpg)

![PALLIATIVE RADIOTHERAPY FOR LIVER
METASTASES
Commonest visceral metastases ~40% with a median OS – 7.5m to 20m
Asymptomatic / Liver dysfunction on labs / frank jaundice with assoc. nausea,
vomiting, pain, anorexia, abd.
distension
Surgery, SBRT, RFA, commonly used for limited mets & systemic chemotherapy or
Chemoembolization / Radioembolization for more numerous mets.
Whole Liver Radiotherapy (WLRT) effective as an aggressive modality for symptom
control
No improvement in OS [ 8-17 weeks in pts receiving liver RT]
RR for pain range from 55 to 80%
Underutilized due to fear of RT induced hepatic toxicity](https://image.slidesharecdn.com/pallrt-230519234310-2a401ed2/85/Pall-RT-pptx-31-320.jpg)
![Series RT schedule
(Gy/fraction #)
No. of
patients
Design Outcomes / comments Median survival
Bydder et
al. 2003 [6],
10/2 28 Prospective
multi-institutional
TROG
Improvement in baseline symptoms in ∼50–66%,
Pain relief in 65%
7% Acute Gd 3/4 toxicity
No late toxicity
10 wk
Leibel et al.
1987 [5] *,
21/7* 214 Randomized ±
Misonidazole
multi-institutional
RTOG
Pain relief in 80% (complete in 54%)
Performance status improved in 28%
Worsening of nausea in 6%
No difference with misonidazole
17 wk
Yeo et al.
2010 [27]
21/7
30/10
(30/15)
10 Further chemotherapy became possible in 40%
Estimated <5% risk of RILD [28]
– May exceed 5% risk of RILD and delivery in 15
fractions may be safer [28]
8 wk
Russell et al.
1993 [26]
27 – 30 / 18 -20
(1.5 Gy per fxn bid)
173 Dose escalation,
multi-institutional
RTOG
6% Acute Grade 3/4 toxicity
10% late liver injury at 33 Gy
No advantage with higher dose
17 wk
Edyta et al.
2015
17Gy/10 fx median
dose & median
dose/fx was 1.8Gy
26 Retrospective Symptom response rates -100% @ 4 wks & 28% @ 3
mths
Pain loss – 28%
01 yr survival - 39%
Gd 3 toxicity – 3%
19.5 wk
• Acute effects of WLRT can include nausea, diarrhea, and a temporary exacerbation of pain
• RILD can appear at 2-12 weeks as a veno-occlusive dx (5% risk with recommended regimens)
• Conformal Partial Liver RT allows higher treatment doses (24–90 Gy) & RRs (60%) without increasing toxicity
PALLIATIVE RADIOTHERAPY FOR LIVER METASTASES](https://image.slidesharecdn.com/pallrt-230519234310-2a401ed2/85/Pall-RT-pptx-32-320.jpg)

![PALLIATIVE RADIOTHERAPY FOR LIVER
METASTASES
Series RT schedule
(Gy/fraction #)
No. of
patients
Primary site Toxicity Outcomes
Herfarth
et al. 2004
[52]
14–26/1
Dose escalation
35 NR No significant
toxicity
1-y LC, 71%;
1-y OS, 72%
Ambrosino
et al. 2009
[57]
25–60/3 27 CRC (11)
Other (16)
No serious
toxicity
Crude LC
rate, 74%
Lee et al.
2009 [56]
27.7–60/6,
Individualized
dose
68 CRC (40)
Breast (12)
Gallbladder (4)
Lung (2)
Anal canal (2)
Melanoma (2)
Other (6)
No RILD
10% G3/4
acute toxicity
No G3/4 late
toxicity
1-y LC, 71%;
Med survival,
17.6 mo
Goodman
et al. 2010
[58]
18–30/1,
Dose
escalation
26 CRC (6)
Pancreatic (3)
Gastric (2)
Ovarian (2)
Other (6)
No dose limiting
toxicity
2 G2 late GI
toxicity
2 G2 late soft
tissue/ rib
Toxicity 1-y
local
failure, 23%;
2-y OS, 49%](https://image.slidesharecdn.com/pallrt-230519234310-2a401ed2/85/Pall-RT-pptx-34-320.jpg)








