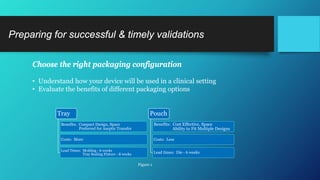
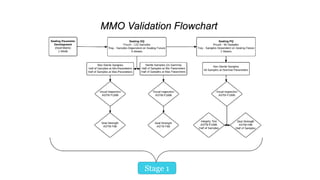
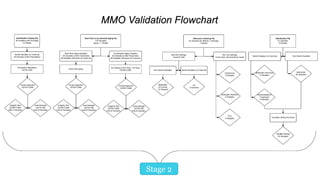
The document discusses common challenges faced in getting medical devices to market quickly and cost-effectively, such as validation processes, resource limitations, and compliance with industry standards. It provides solutions including the selection of appropriate packaging configurations, understanding validation requirements, and considering outsourcing for efficiency. Key takeaways emphasize the importance of avoiding validation failures, leveraging pre-validated packaging solutions, and partnering with experienced packaging providers to enhance quality and speed to market.