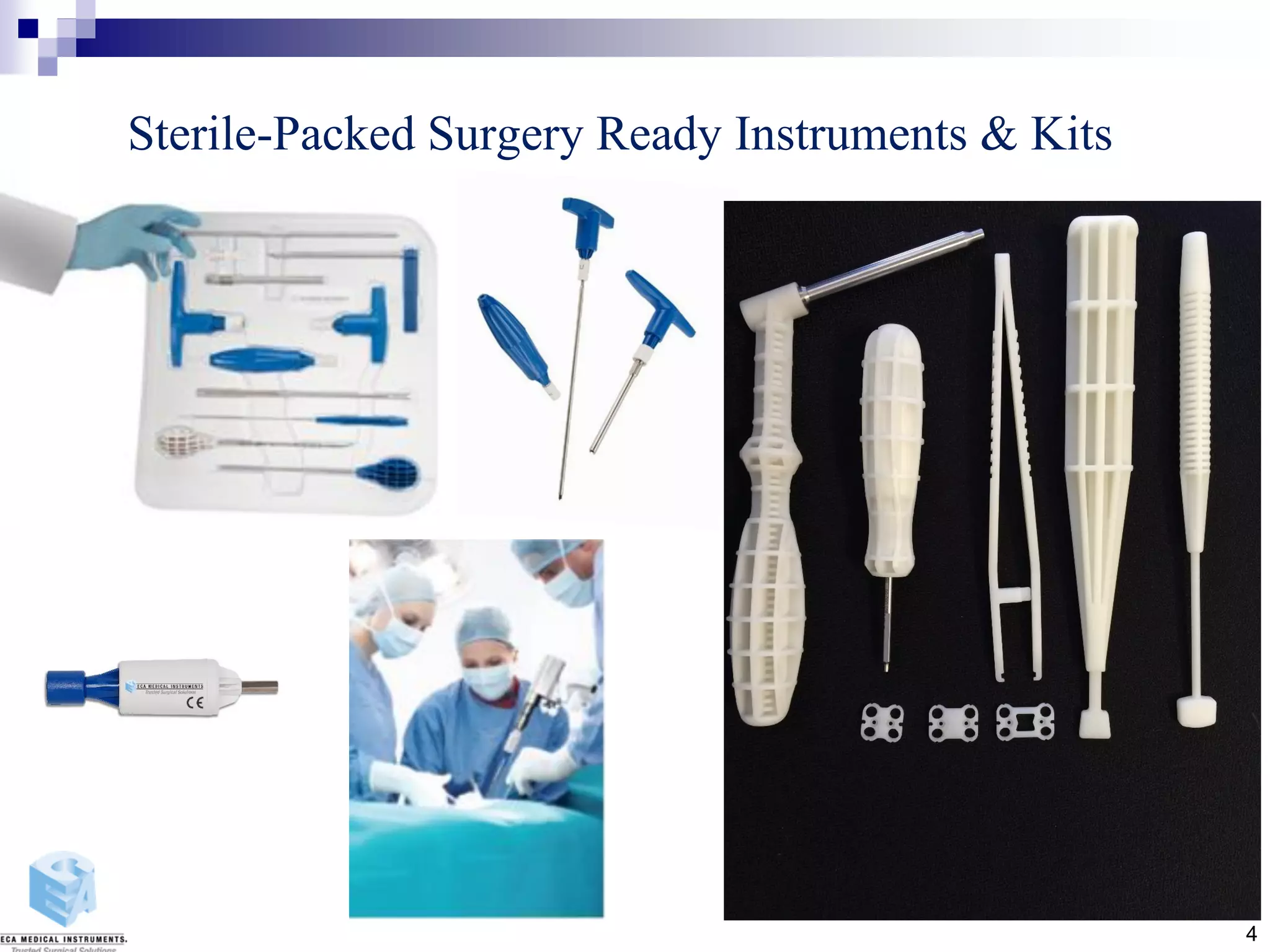
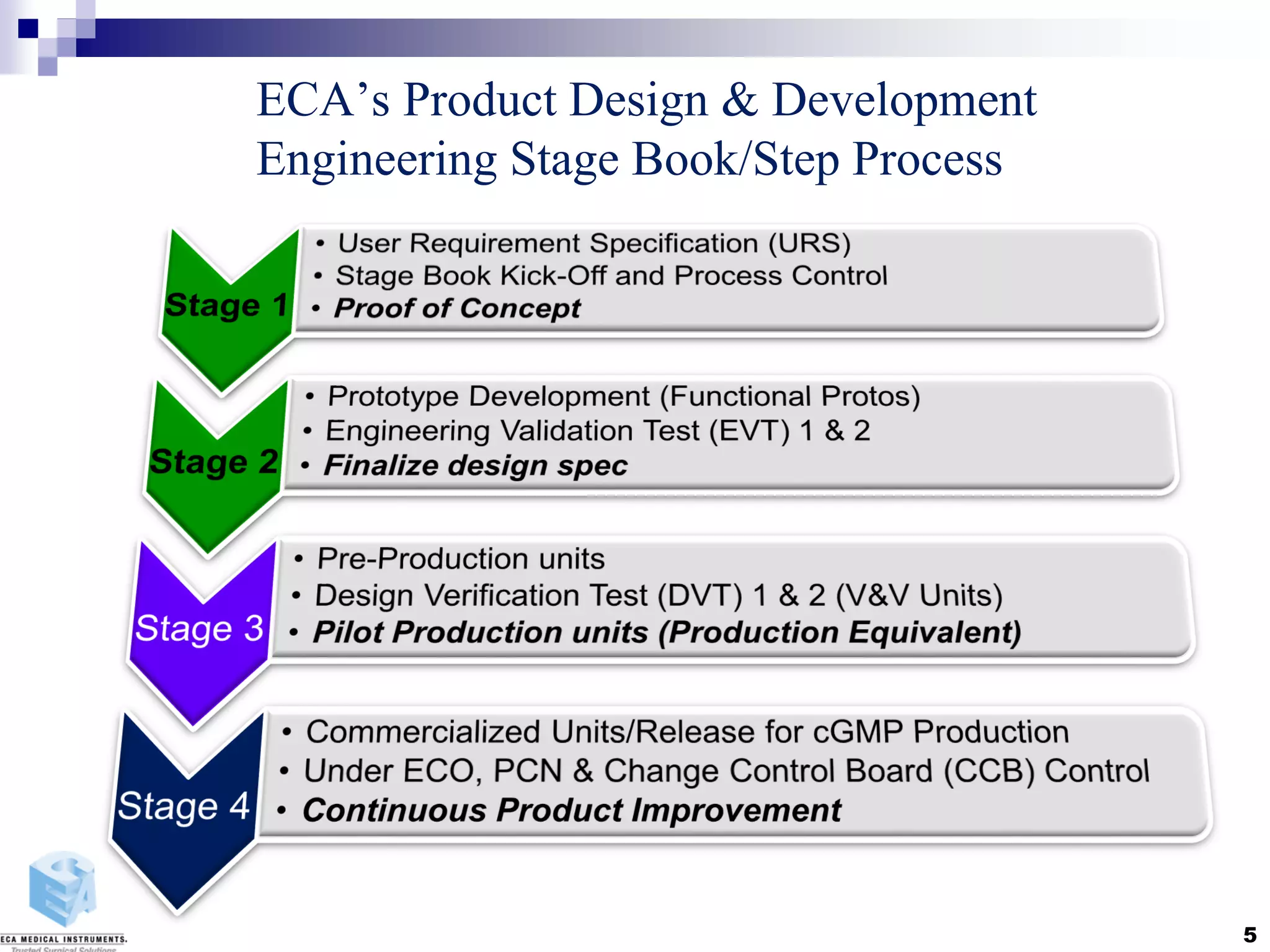
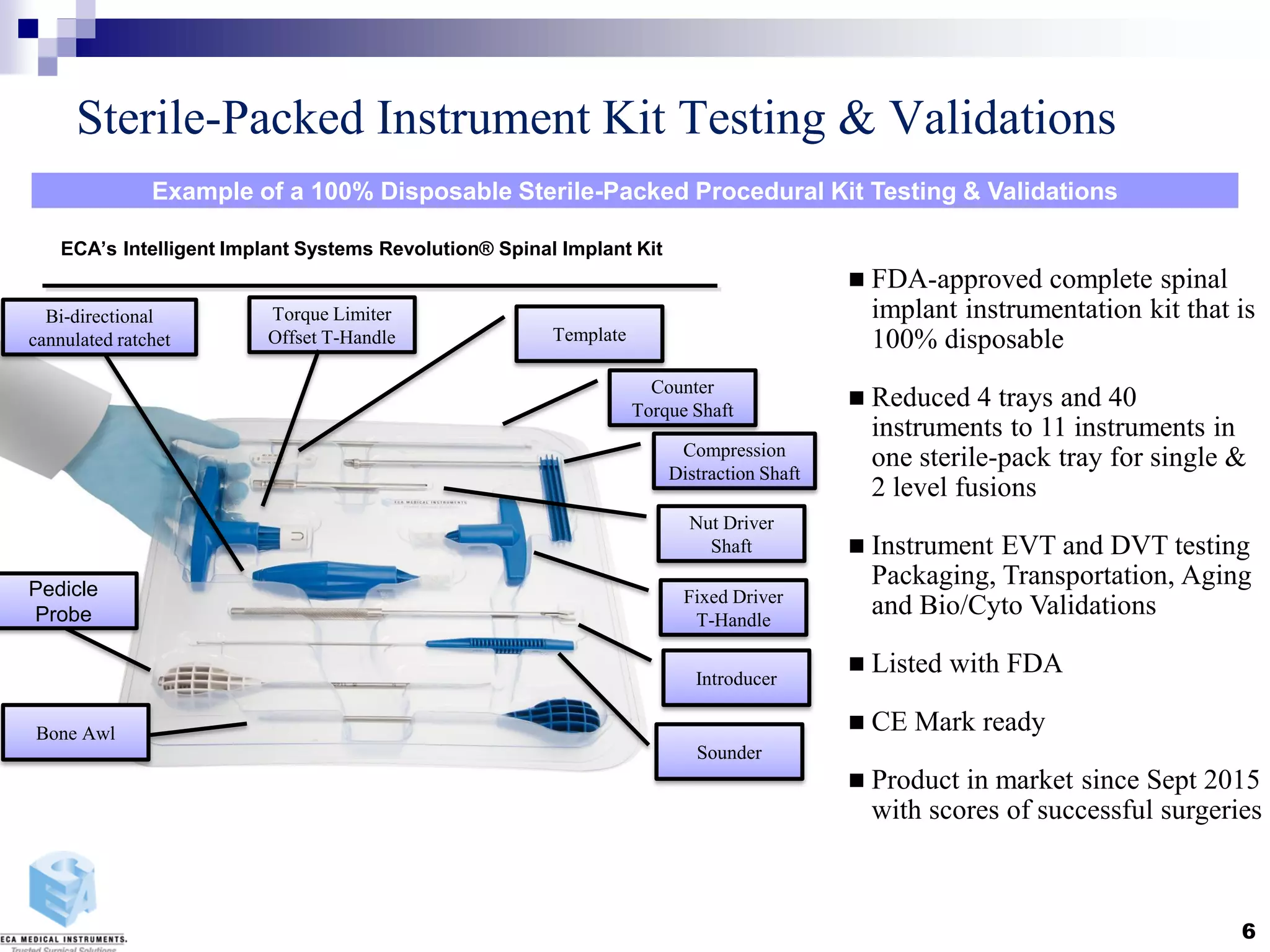
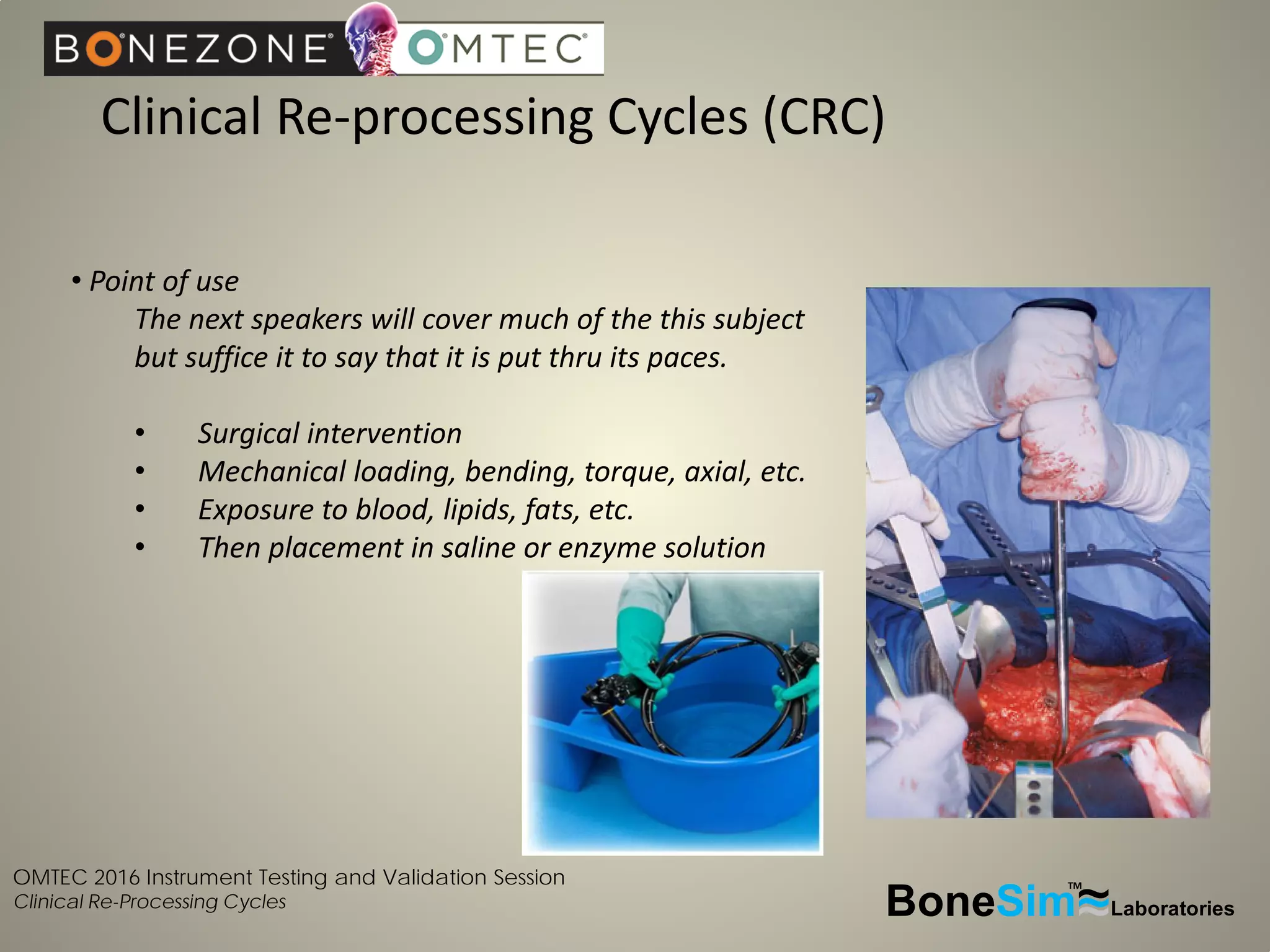
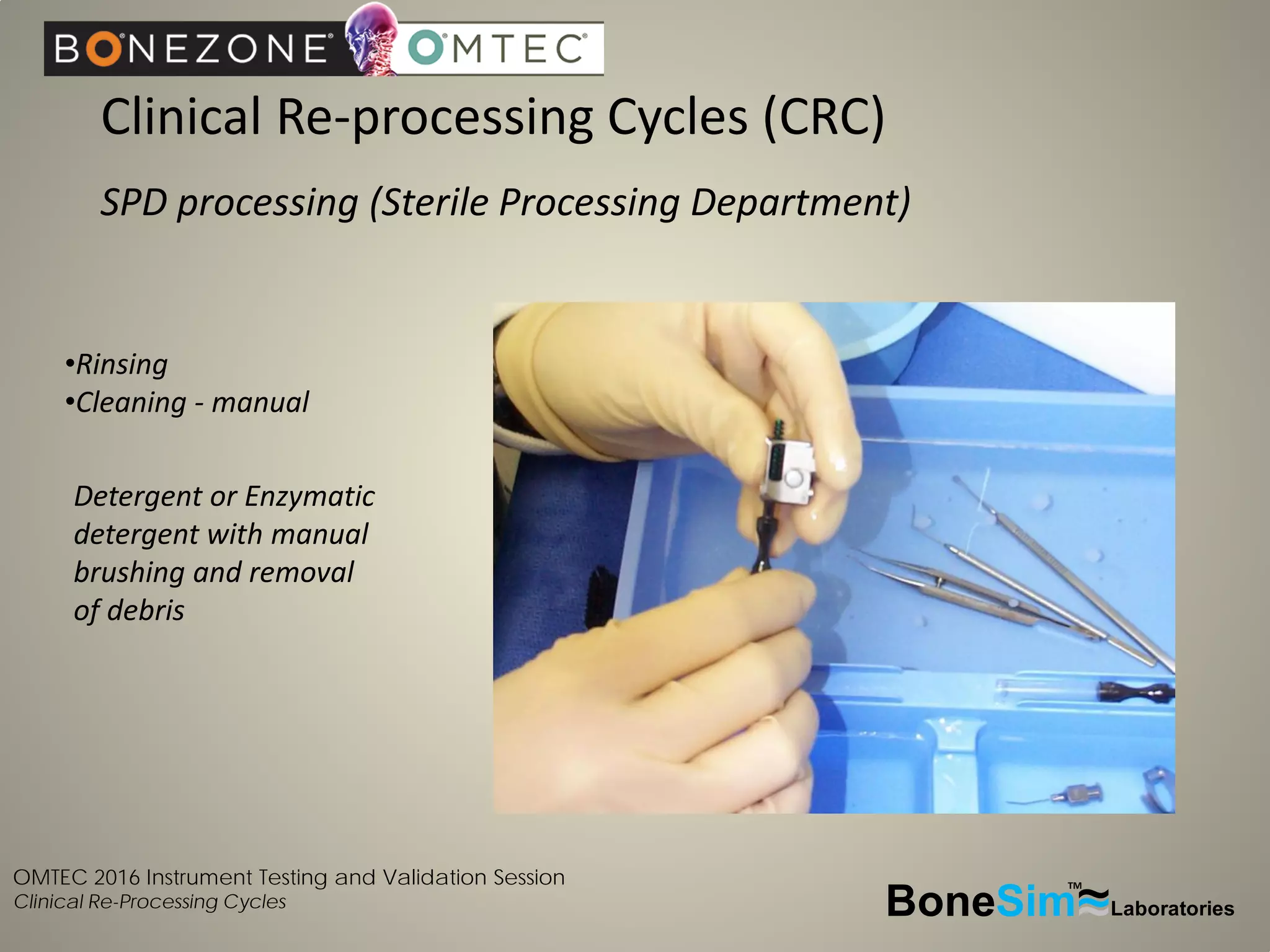
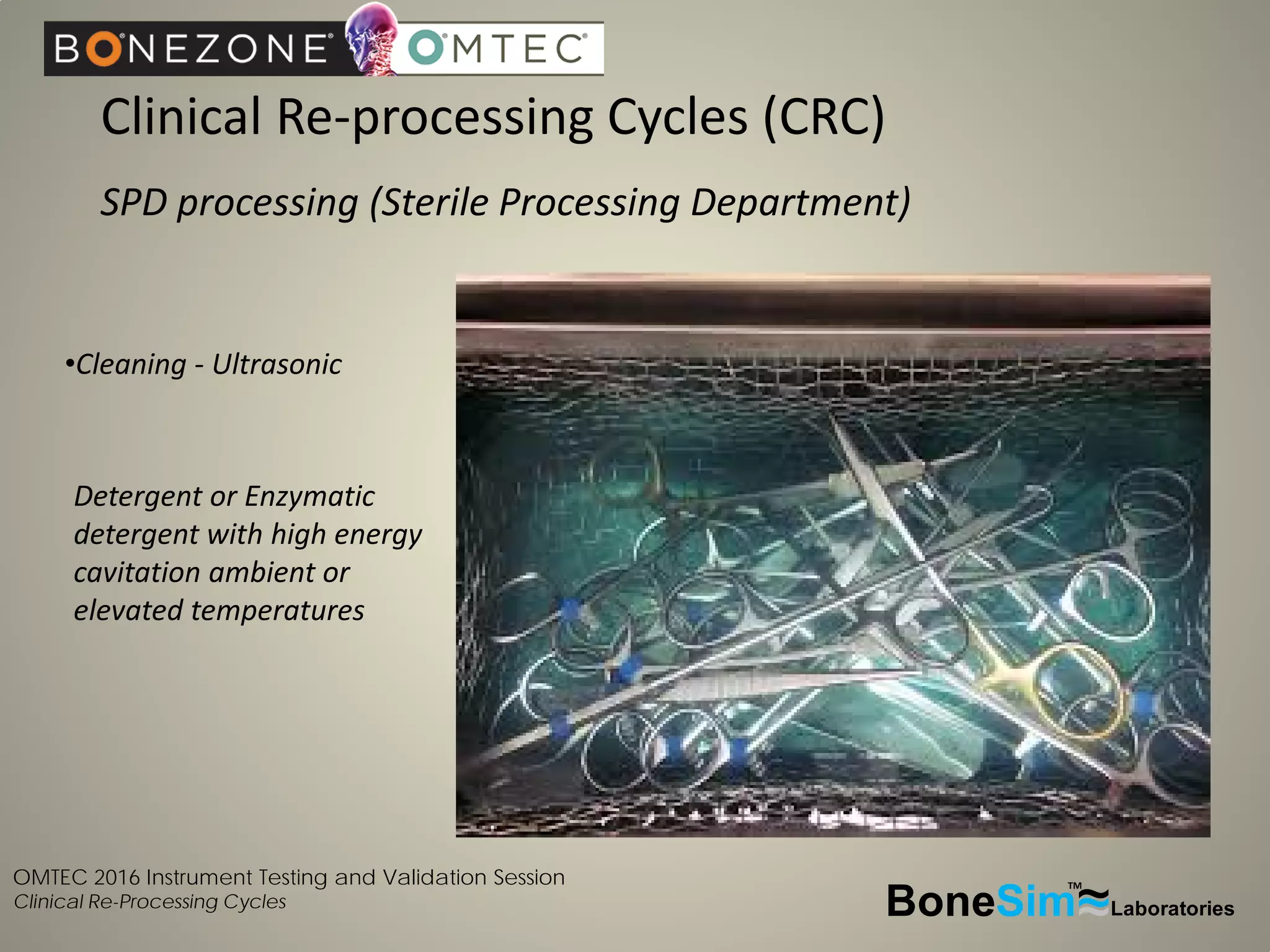
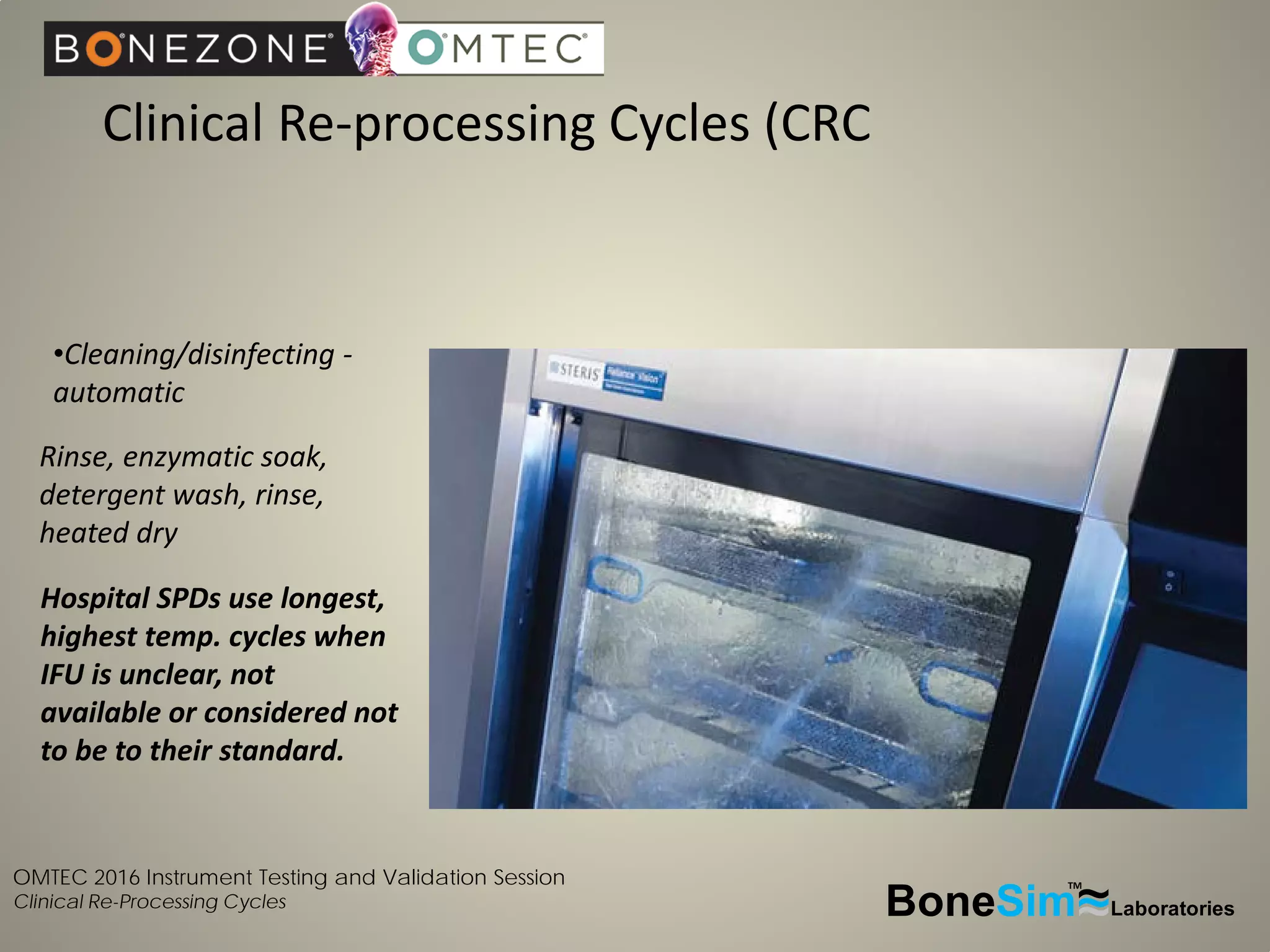
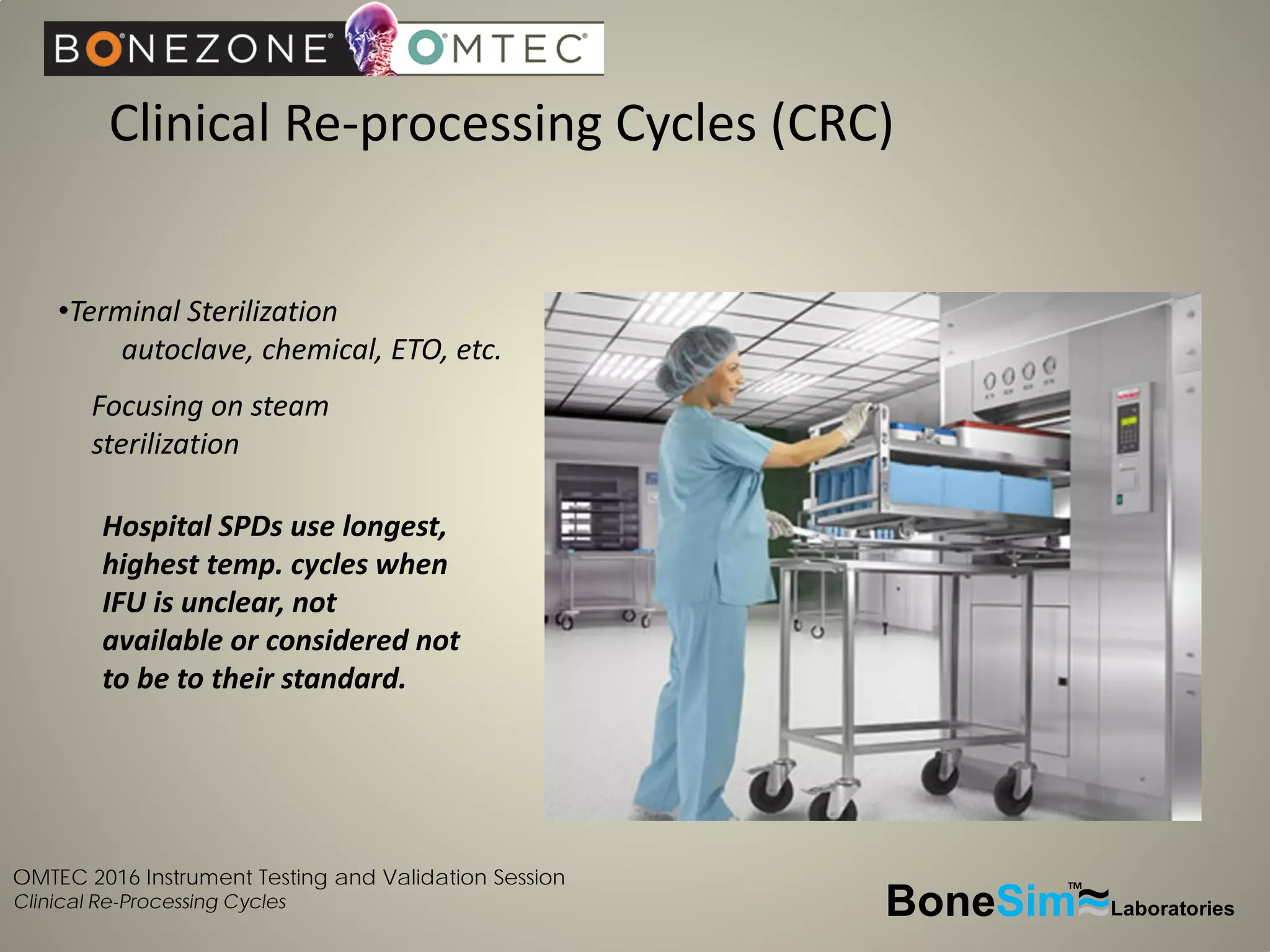
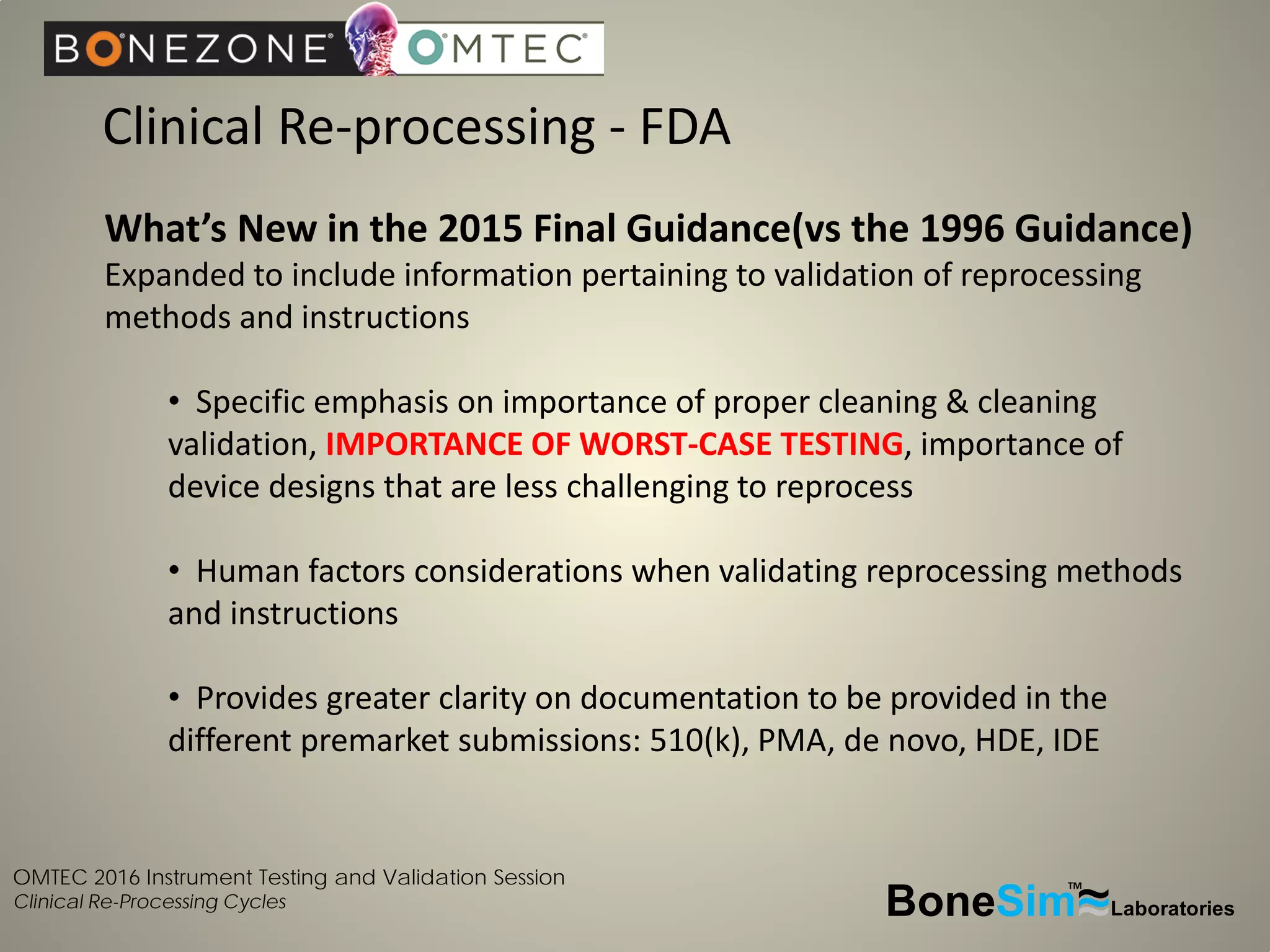
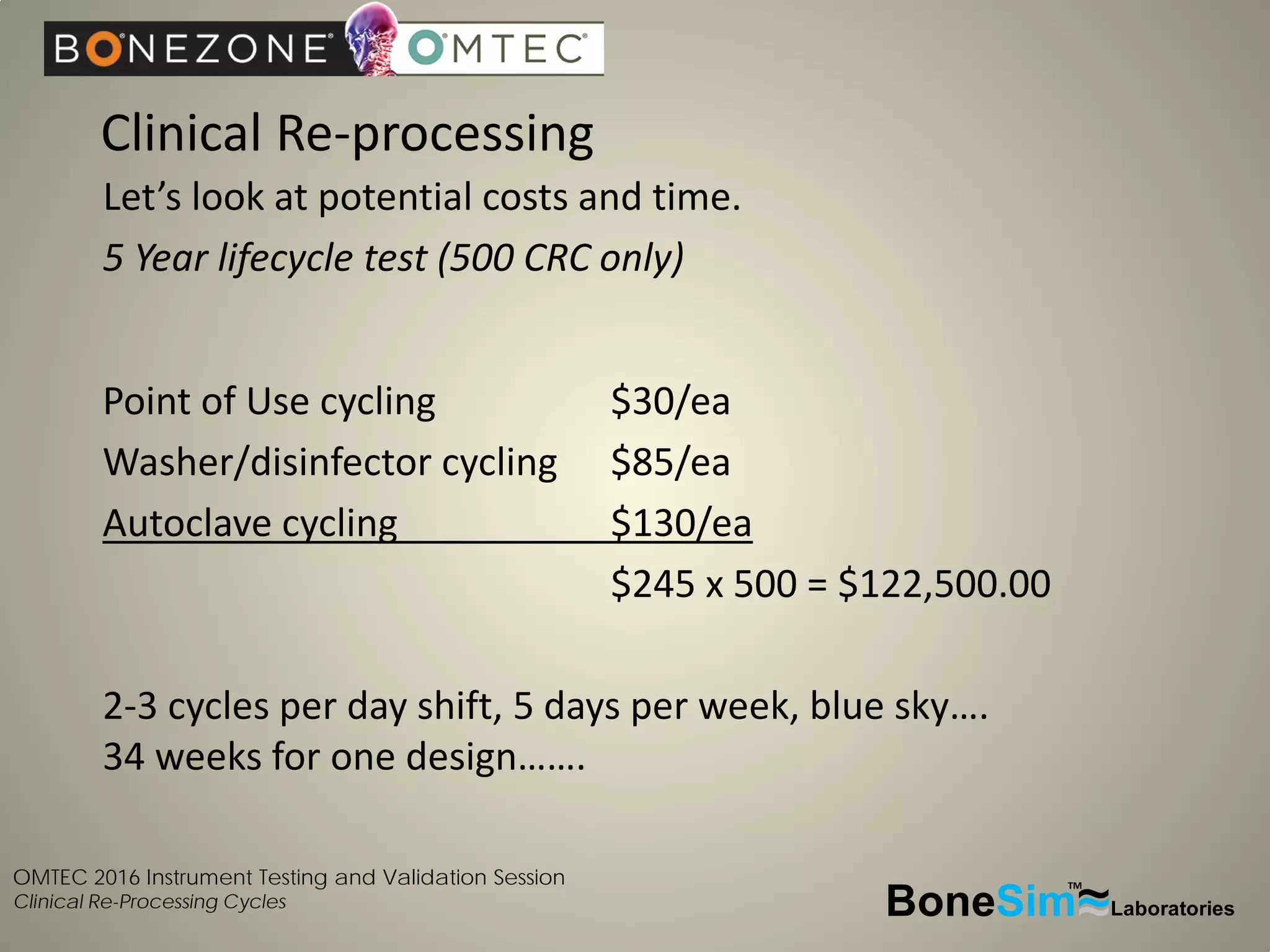
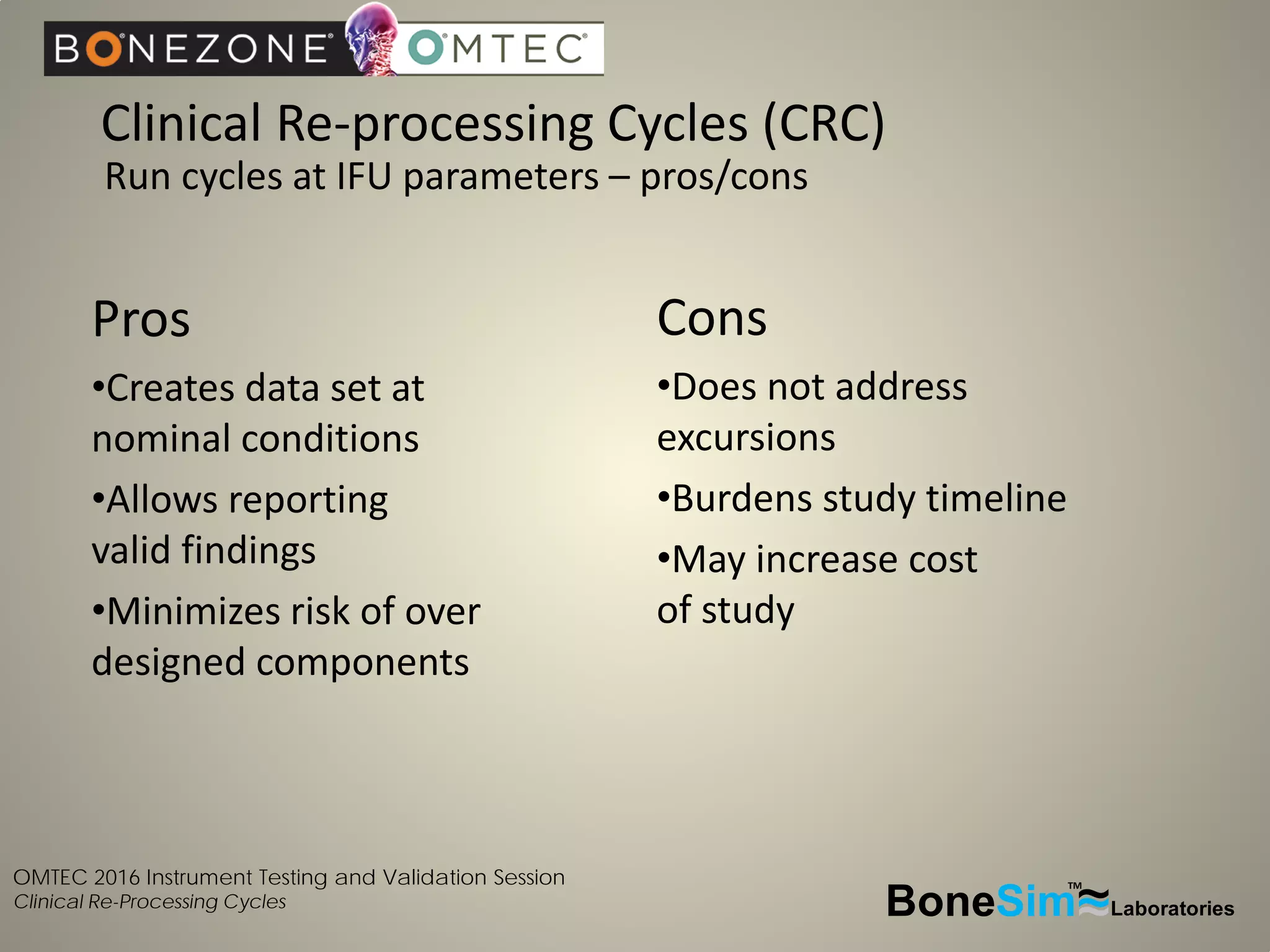
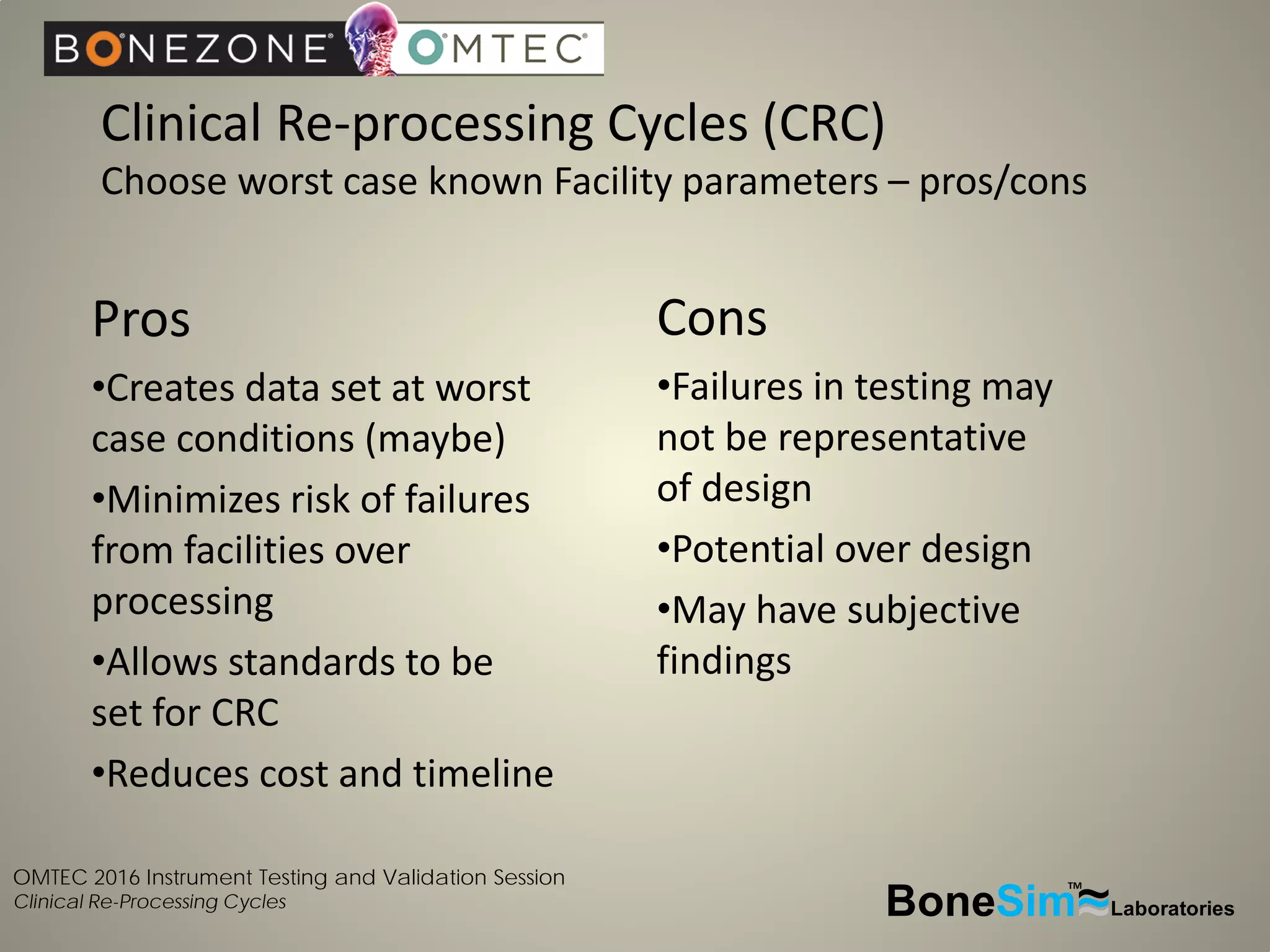
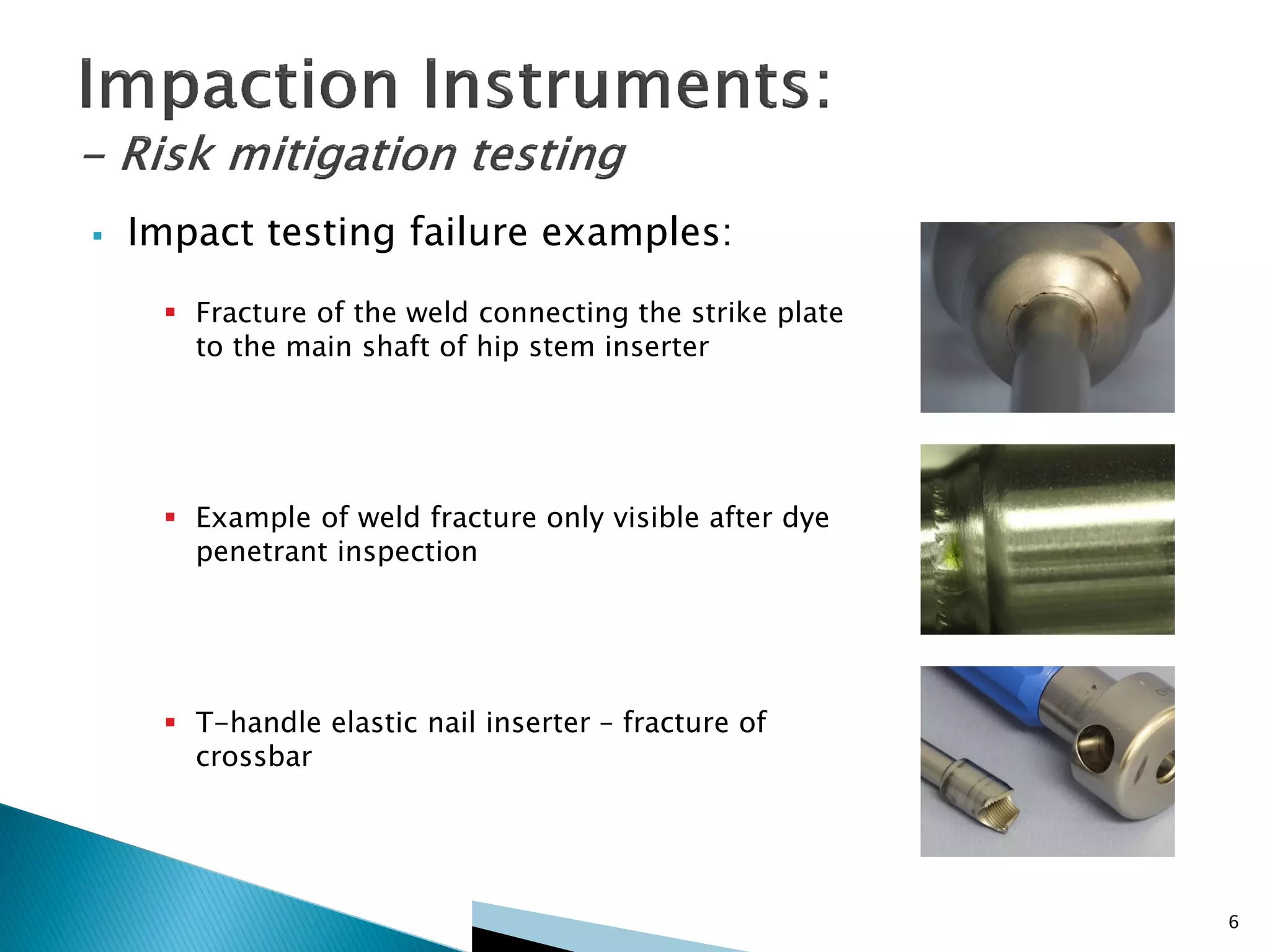
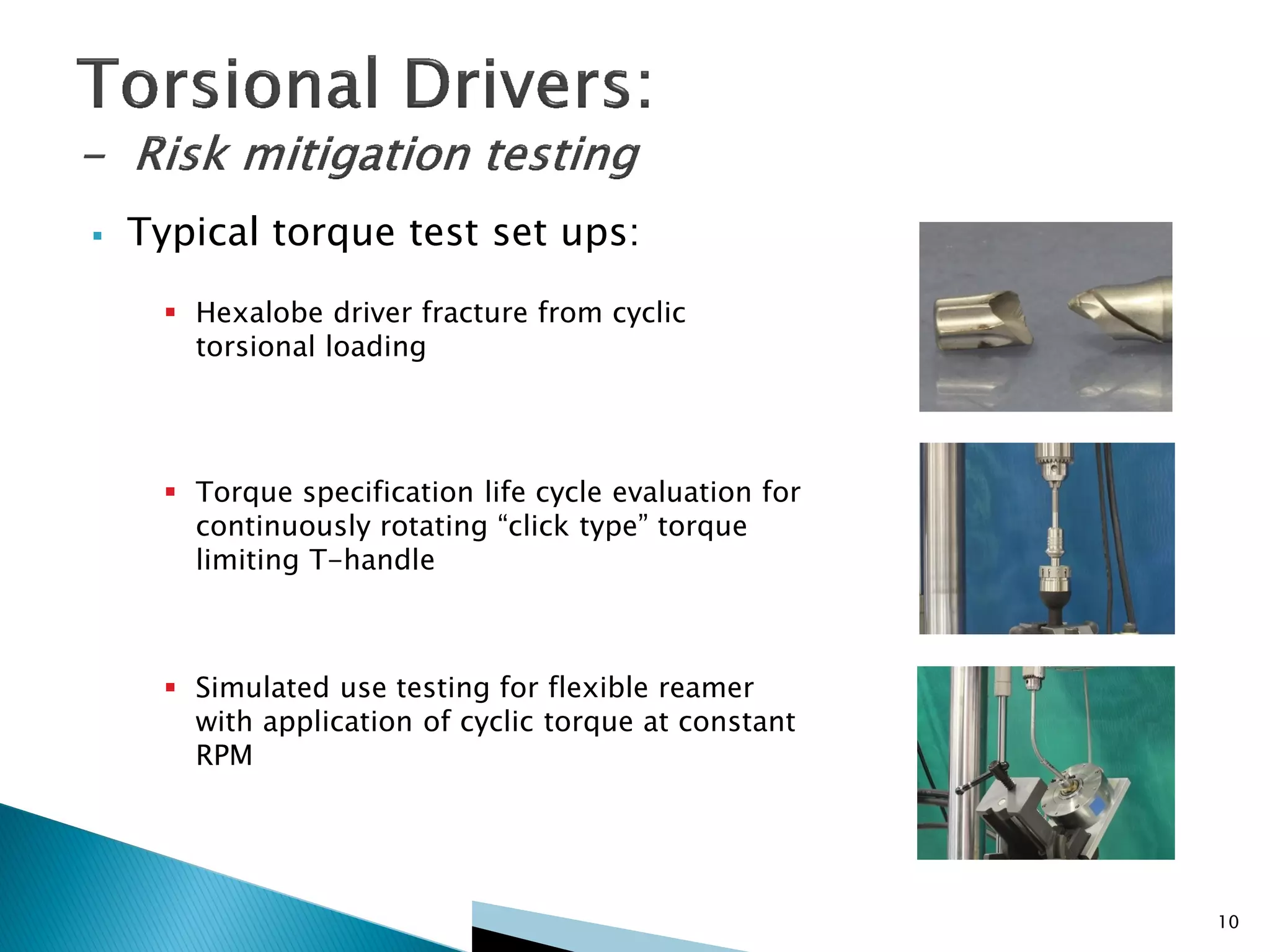
Disposable instruments and procedural kits undergo comprehensive product development and testing to meet quality and regulatory requirements. Extensive validation and verification testing is performed on packaging, sterilization methods, aging, and the instruments themselves to ensure clinical robustness. This includes testing instruments through simulated clinical reprocessing cycles to validate they can withstand repeated cleaning, sterilization, and use over their intended lifetimes. Understanding how instruments will perform after many reprocessing cycles is important for material selection, design, and establishing appropriate cleaning and sterilization instructions.