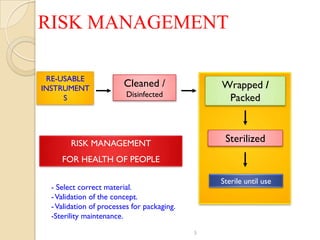
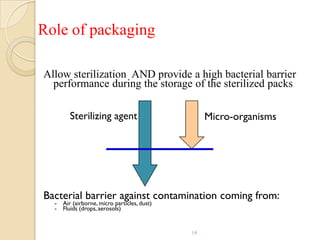
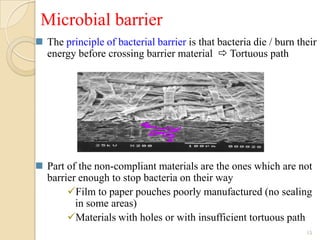
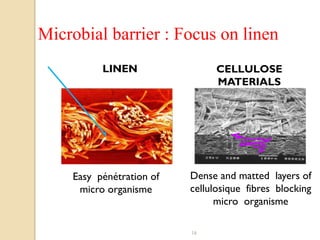
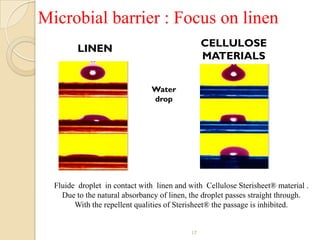
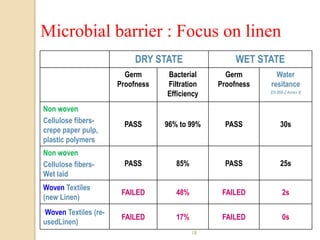
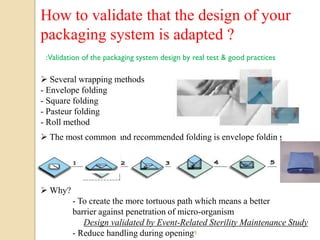
The document discusses sterility maintenance and shelf life management. It covers topics such as material requirements for sterile packaging, packaging system design validation processes, and maintaining sterility and shelf life. Specifically, it emphasizes the importance of using cellulose fibers rather than linen for packaging due to linen's absorbency which can allow contamination. It also notes that double sequential wrapping provides better barrier protection than single wrapping.