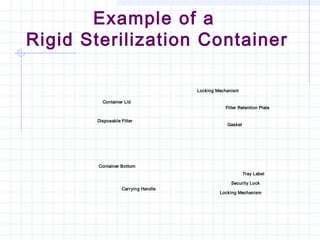
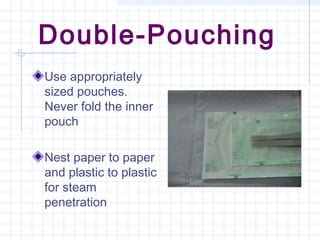
This document provides an overview of sterile packaging and storage. It discusses the objectives of sterile packaging including protecting contents, maintaining sterility, and allowing for aseptic opening. It reviews various reusable and disposable packaging materials like muslin, pouches, and rigid containers. Proper packaging procedures are outlined including preparation, closure methods, labeling, and storage standards. Maintaining sterility through careful handling and following event-related concepts is emphasized.