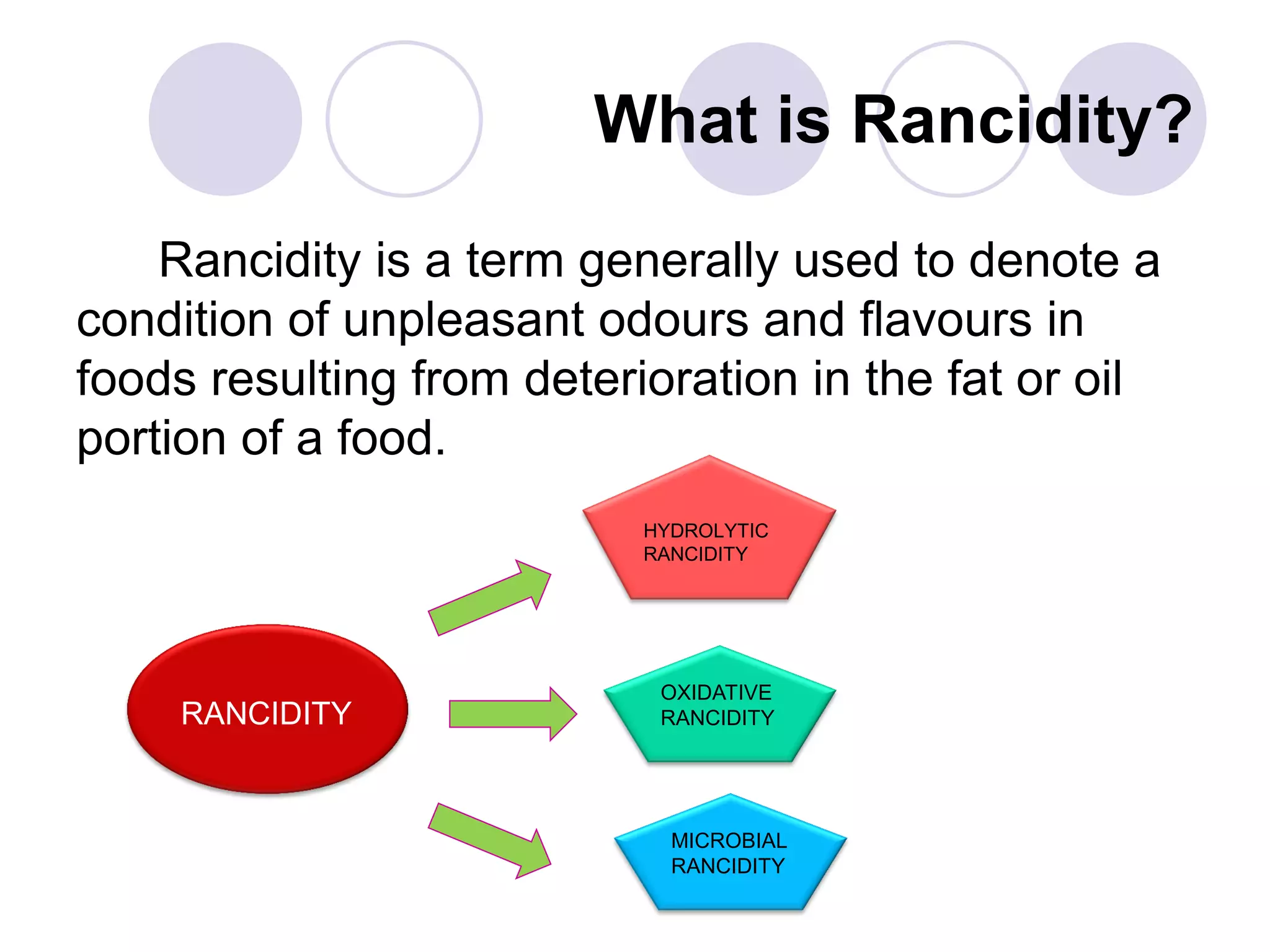
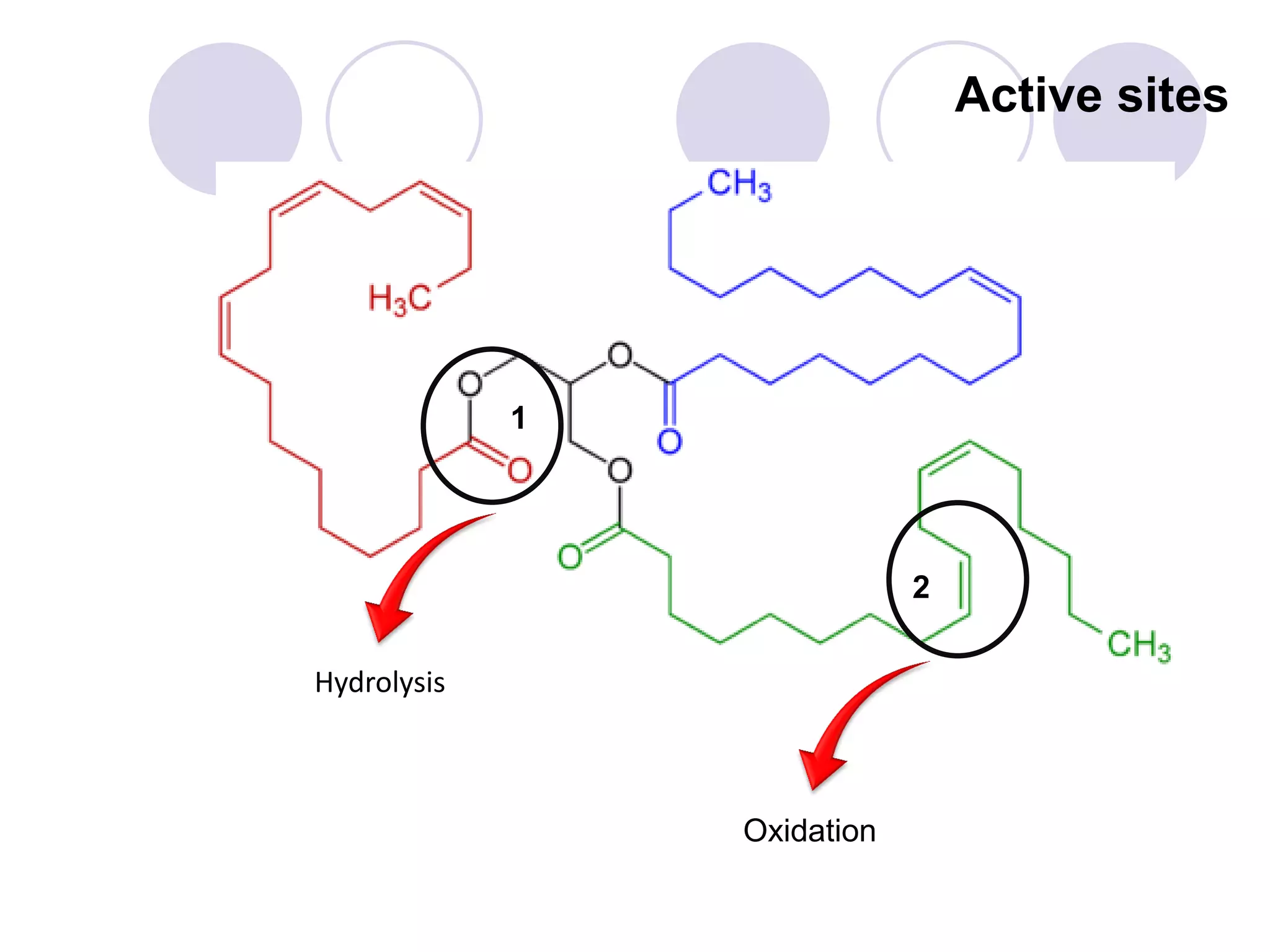
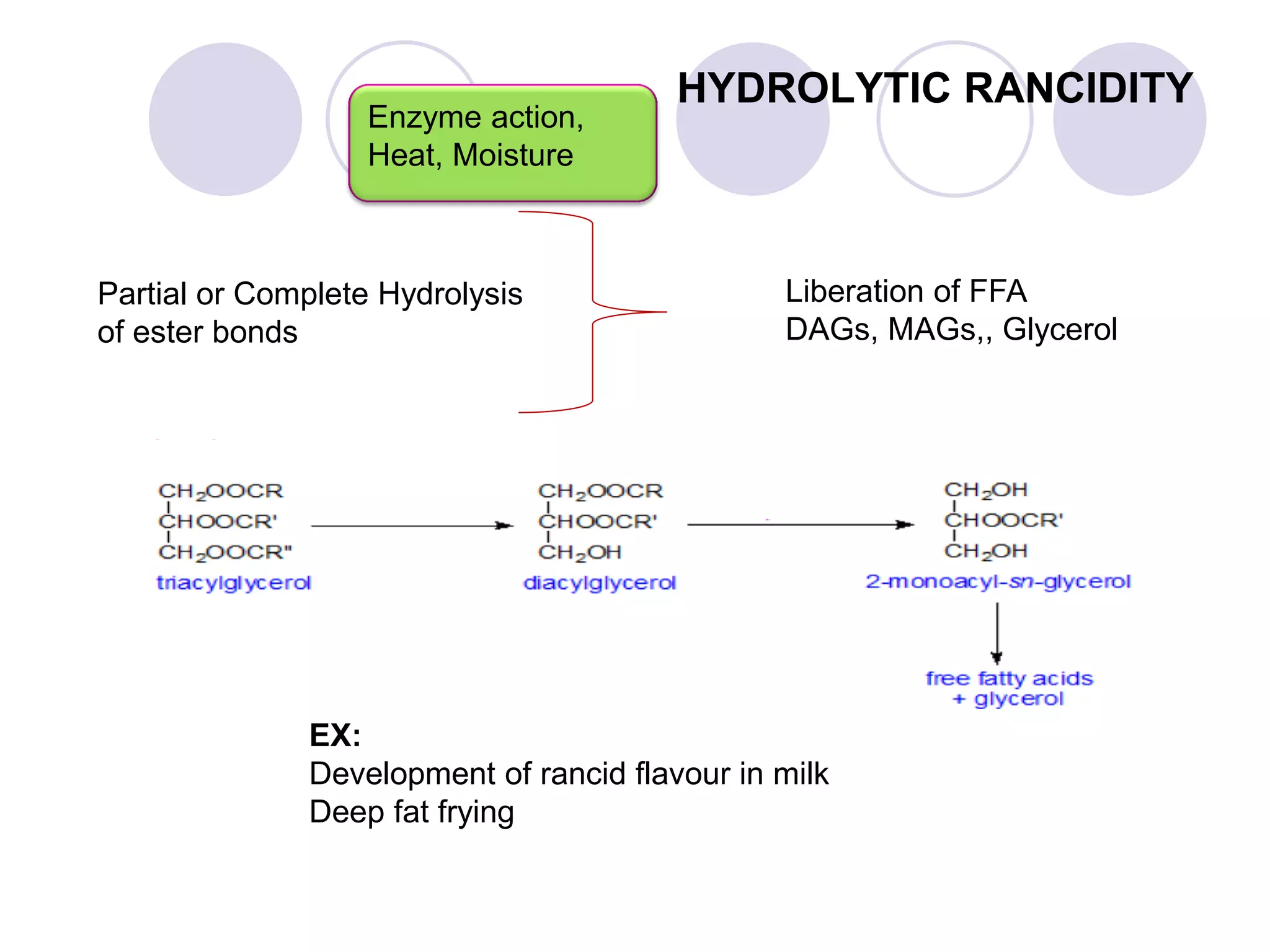
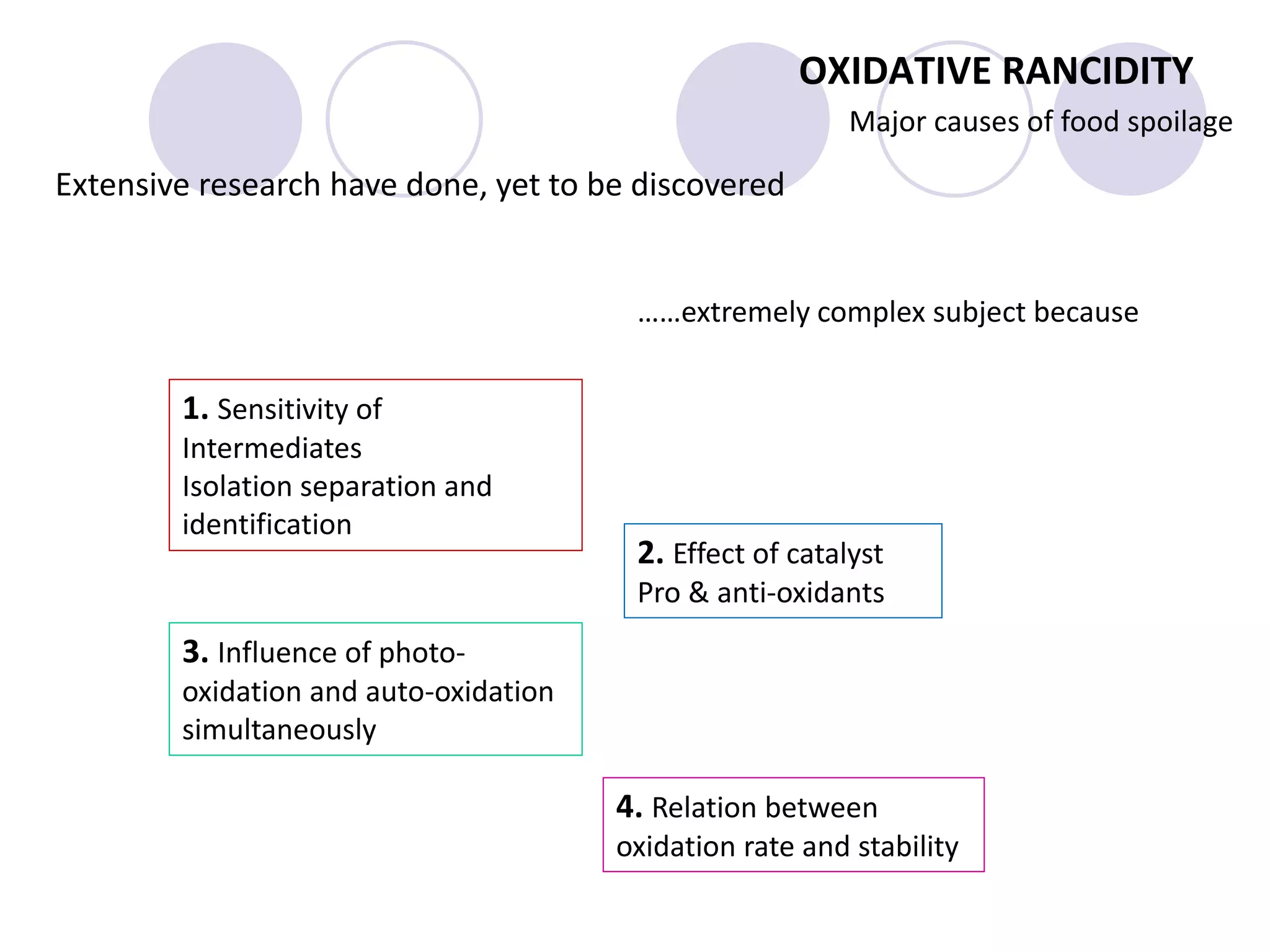
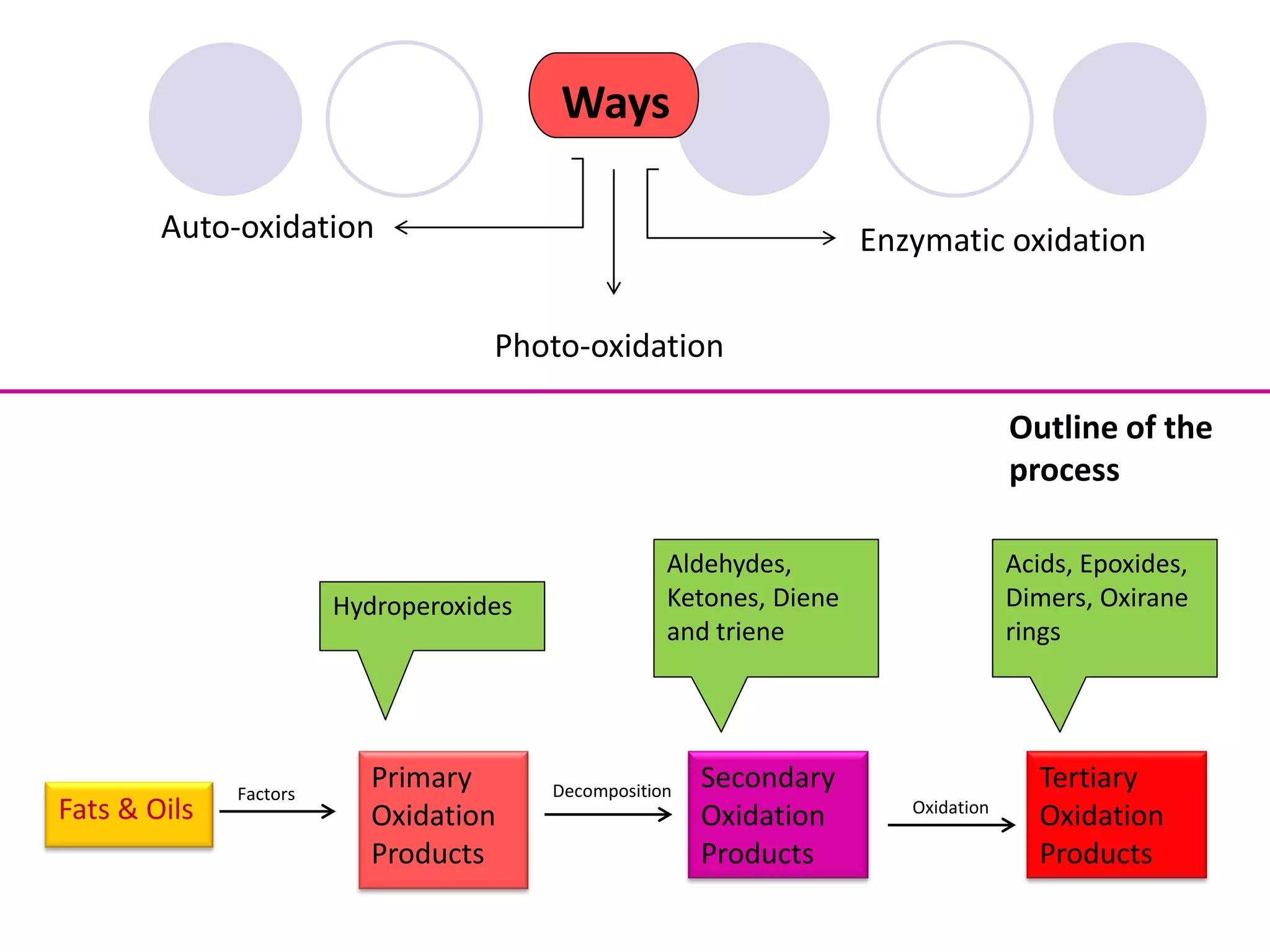
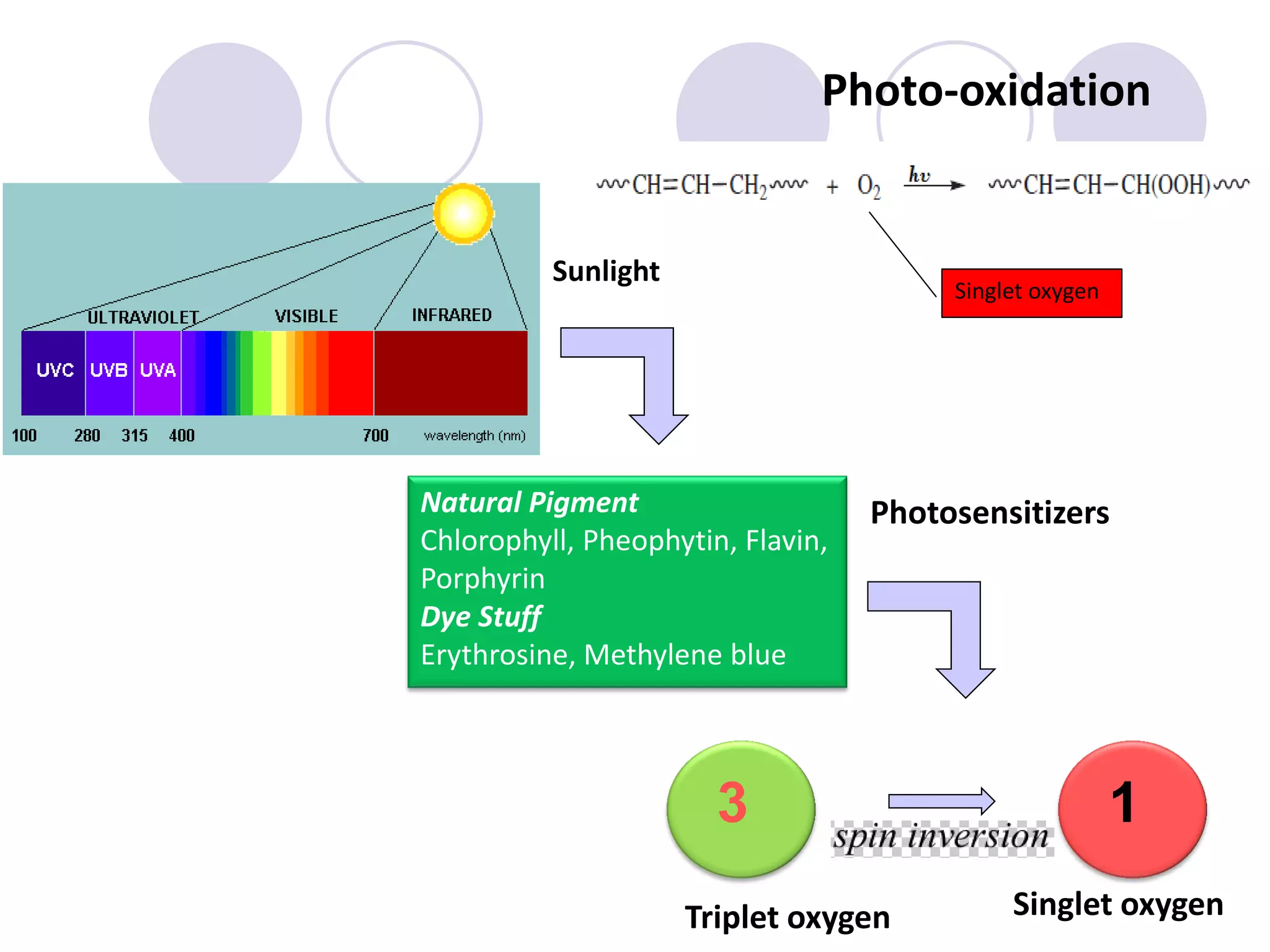
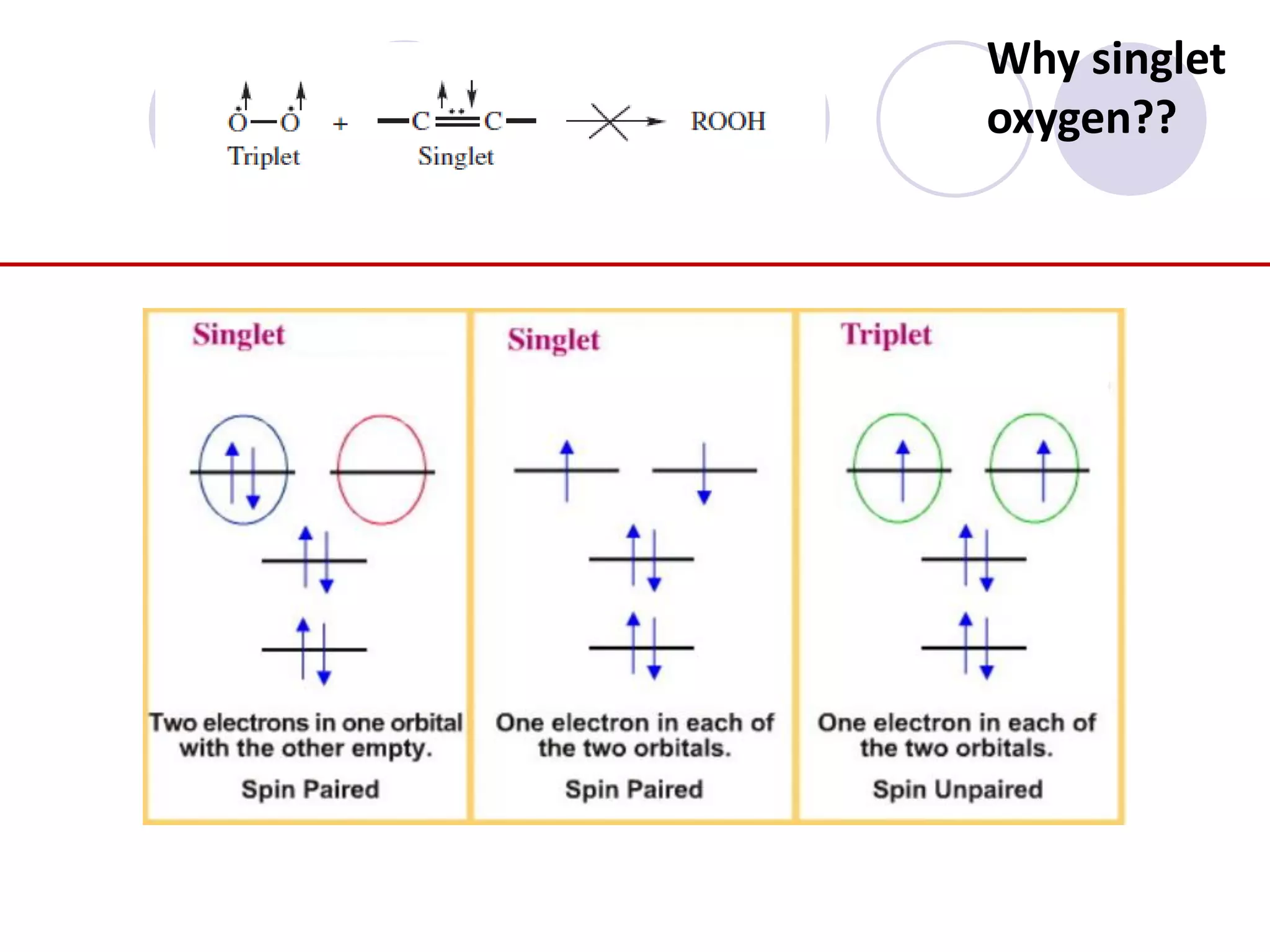
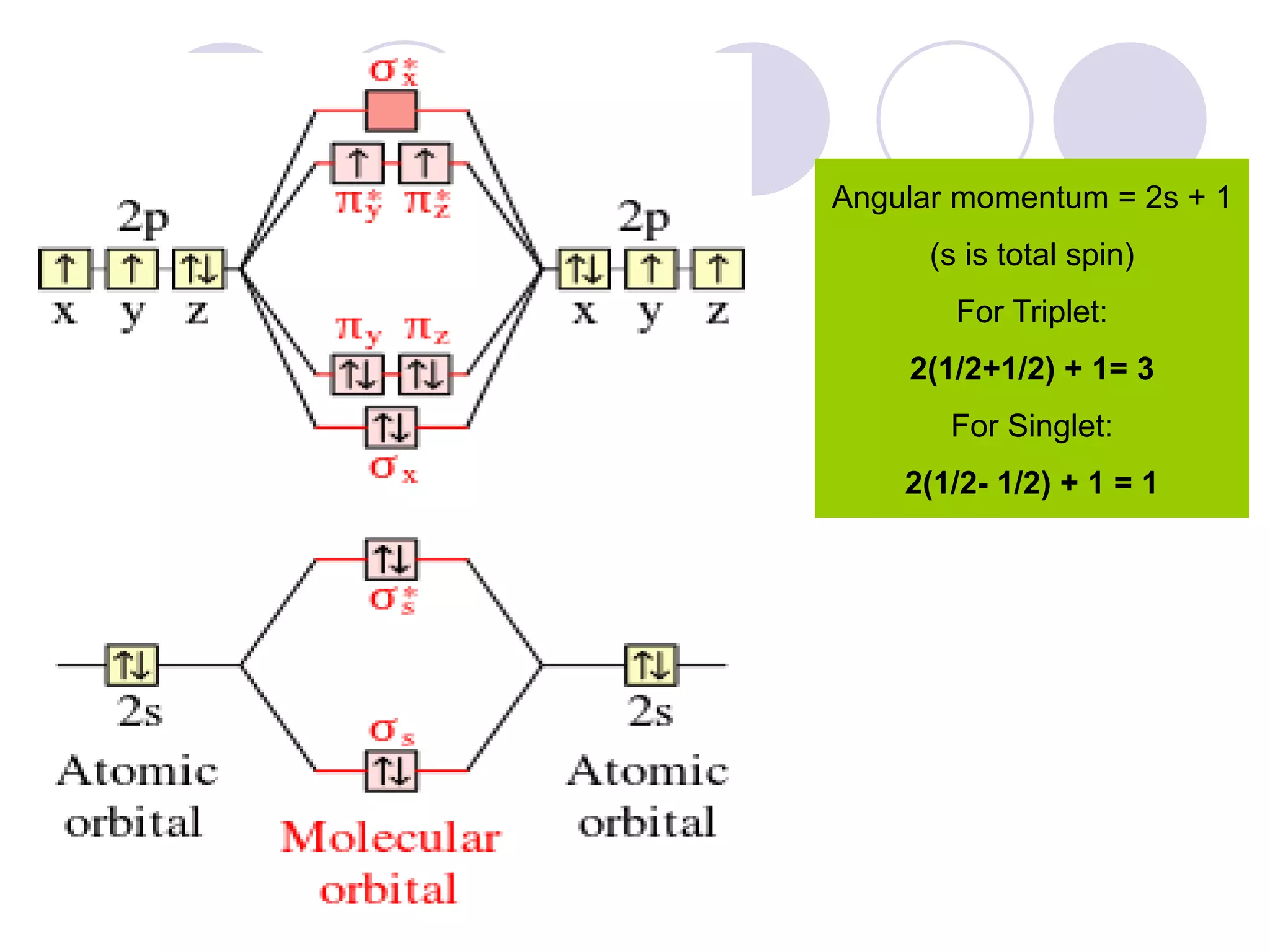
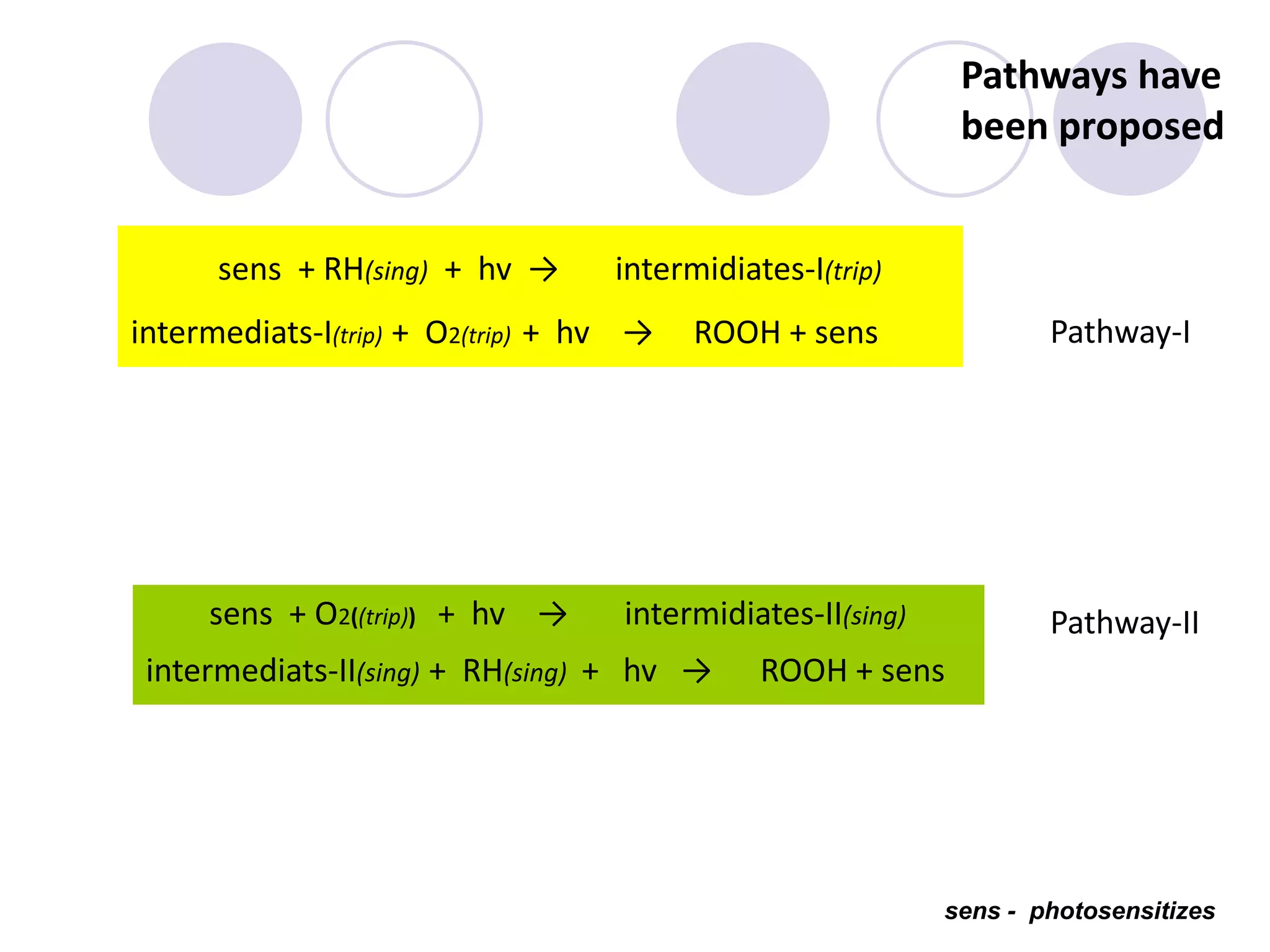
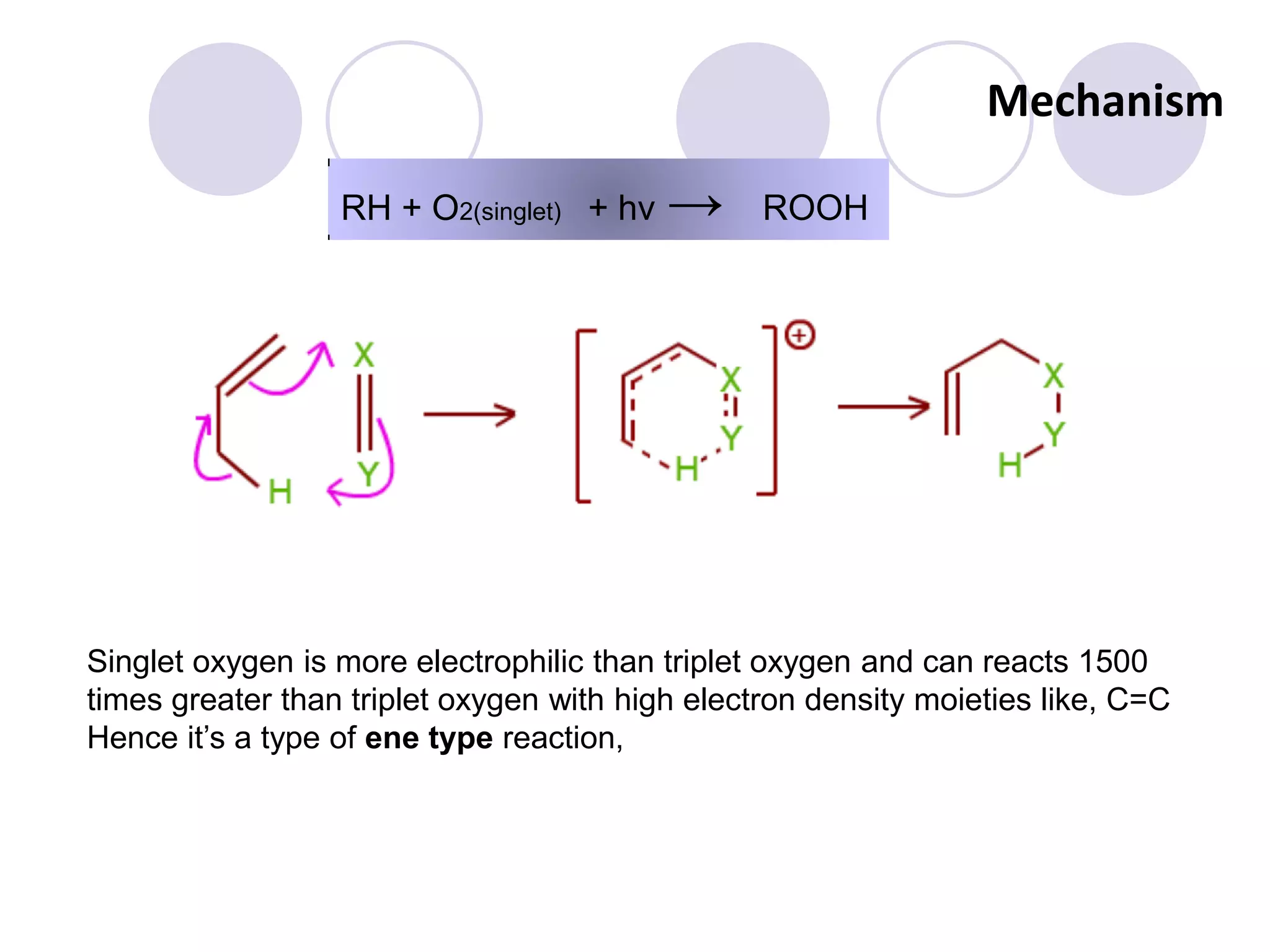
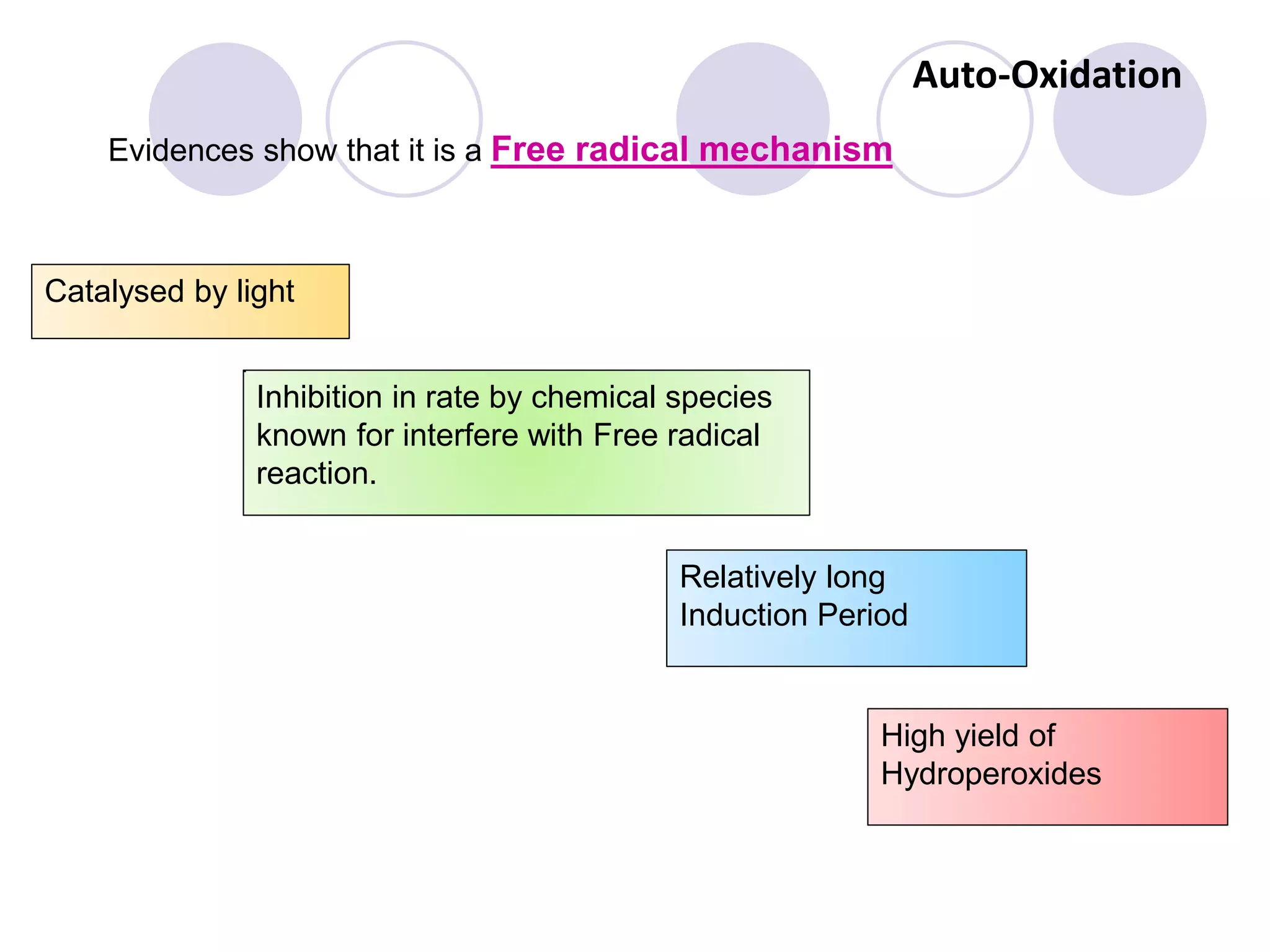
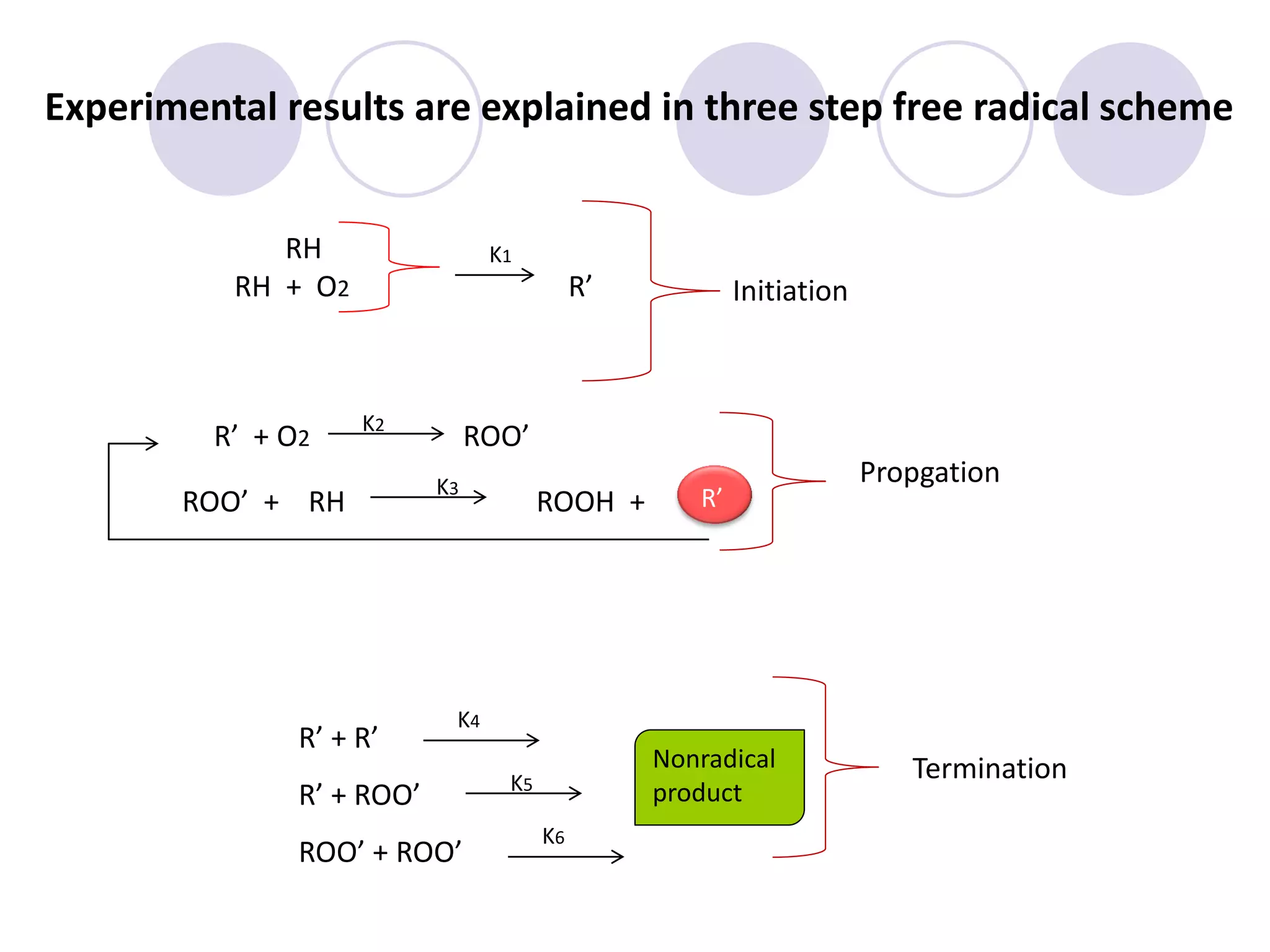
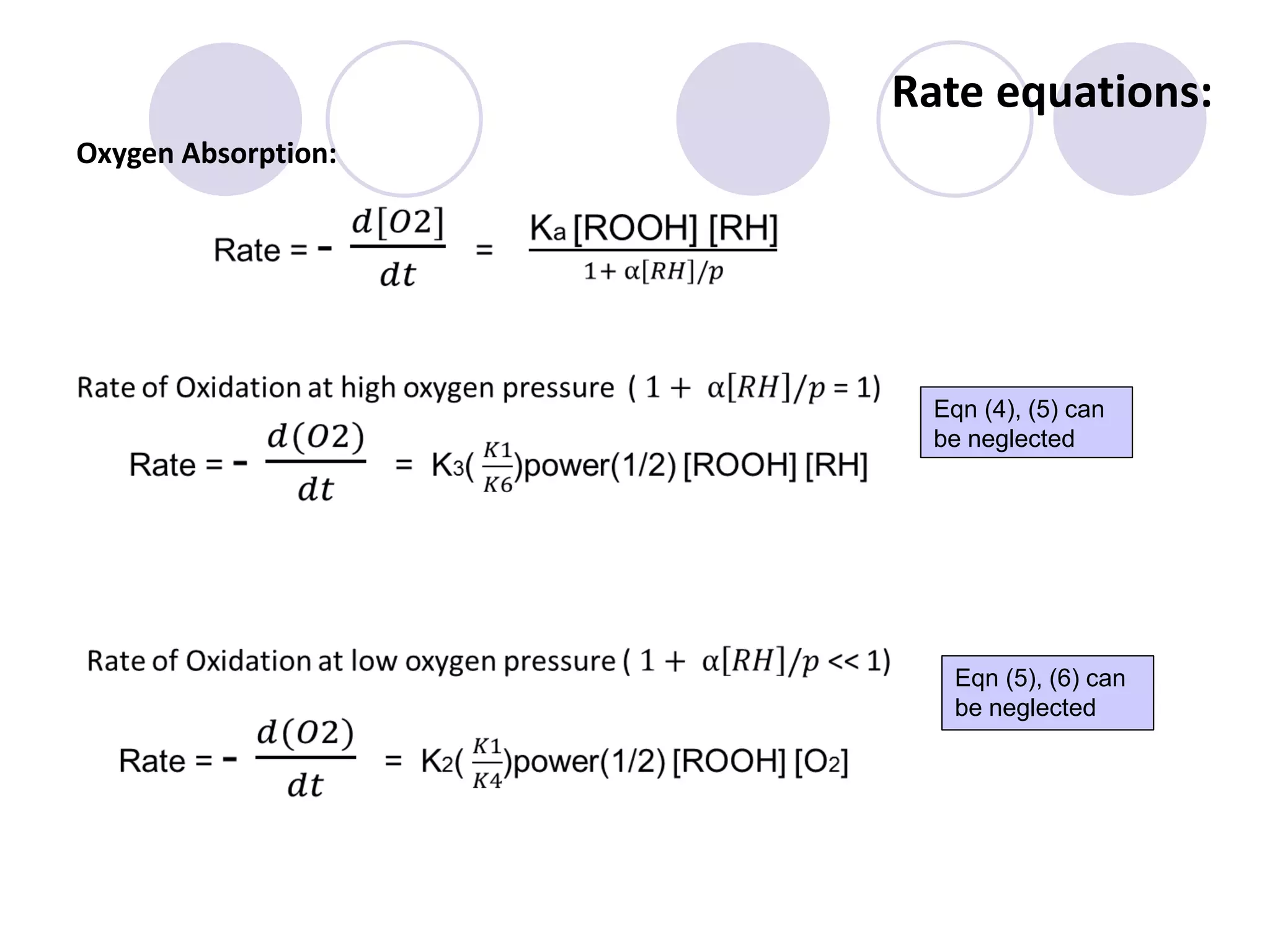
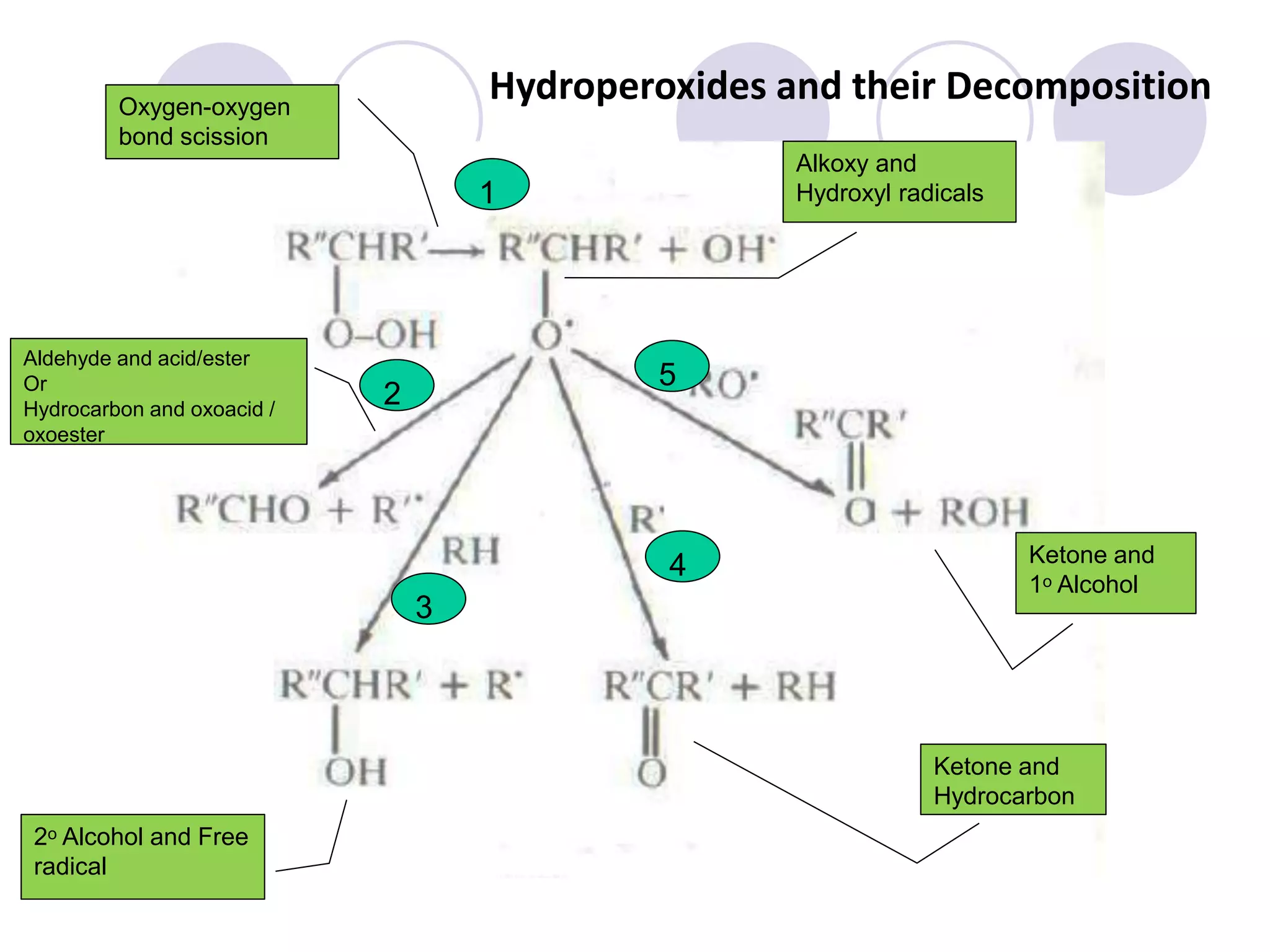
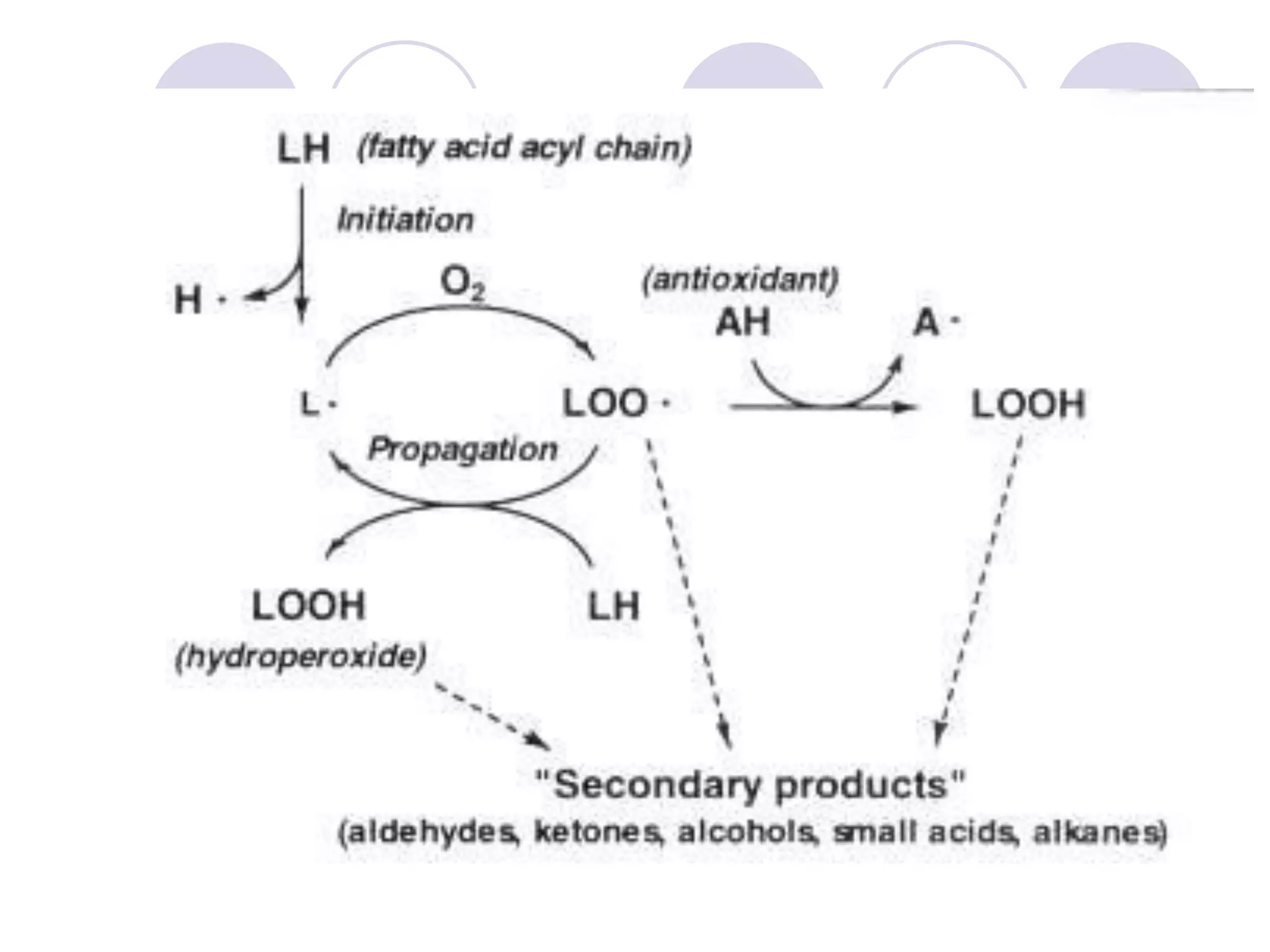
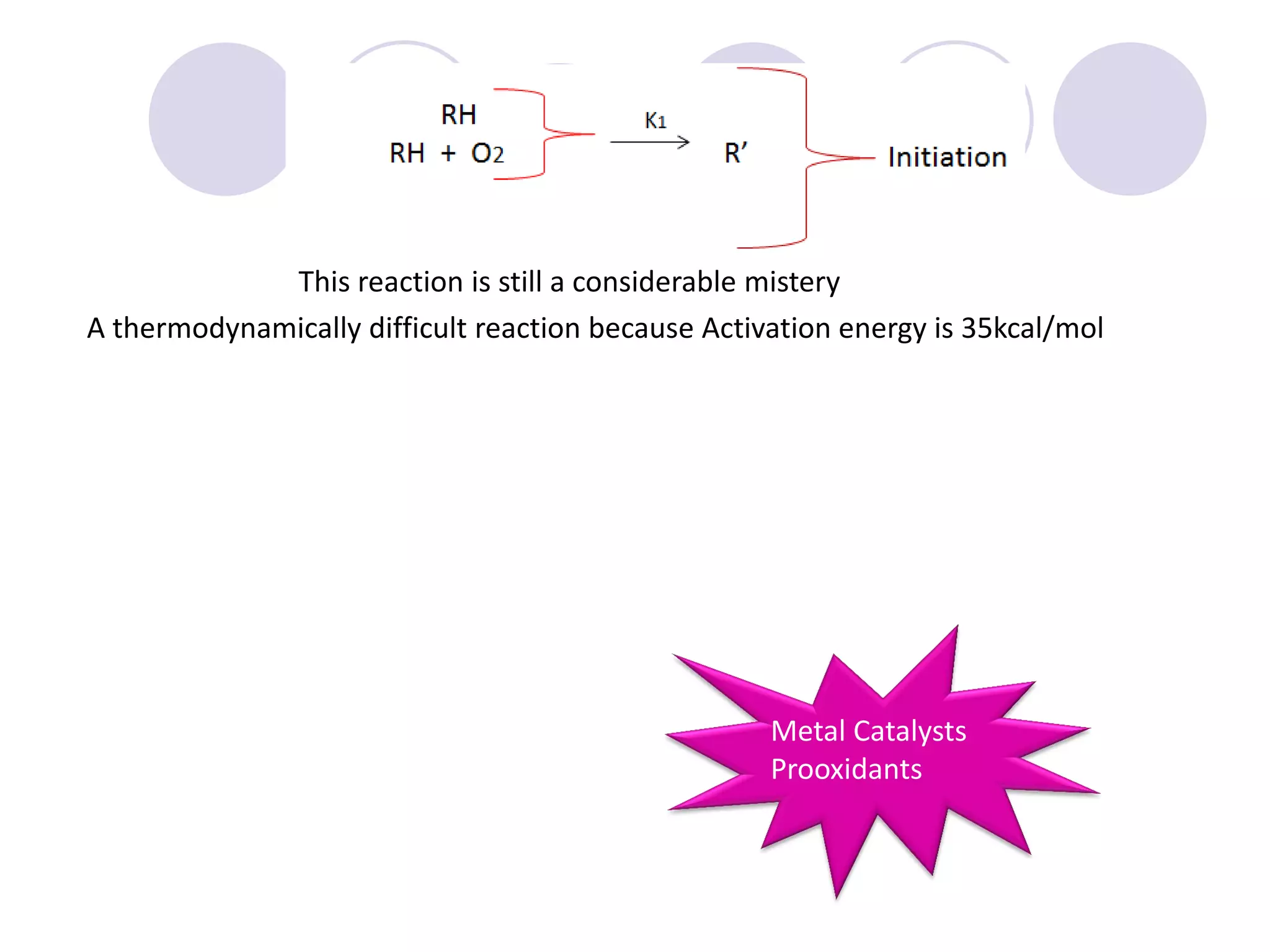
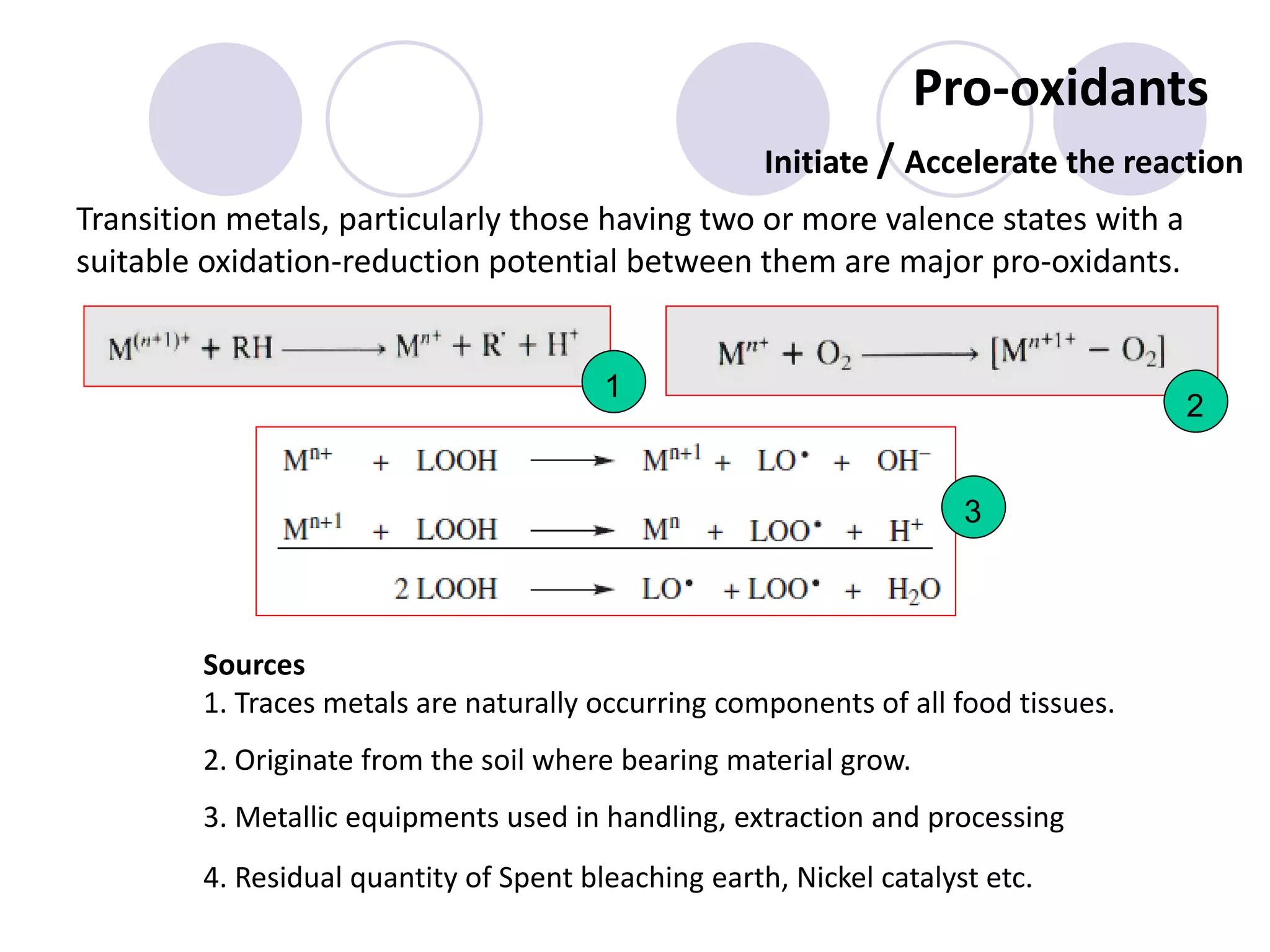
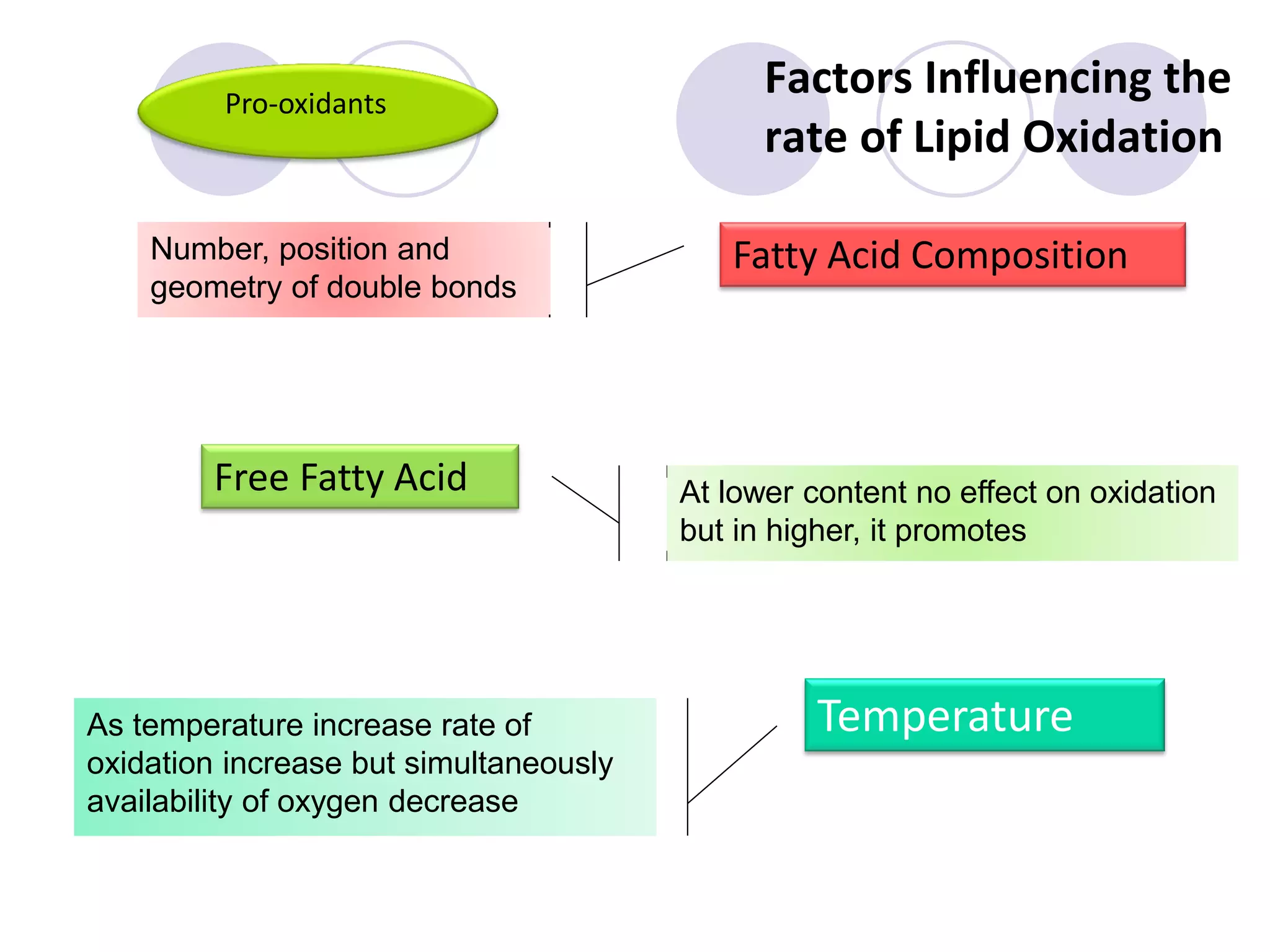
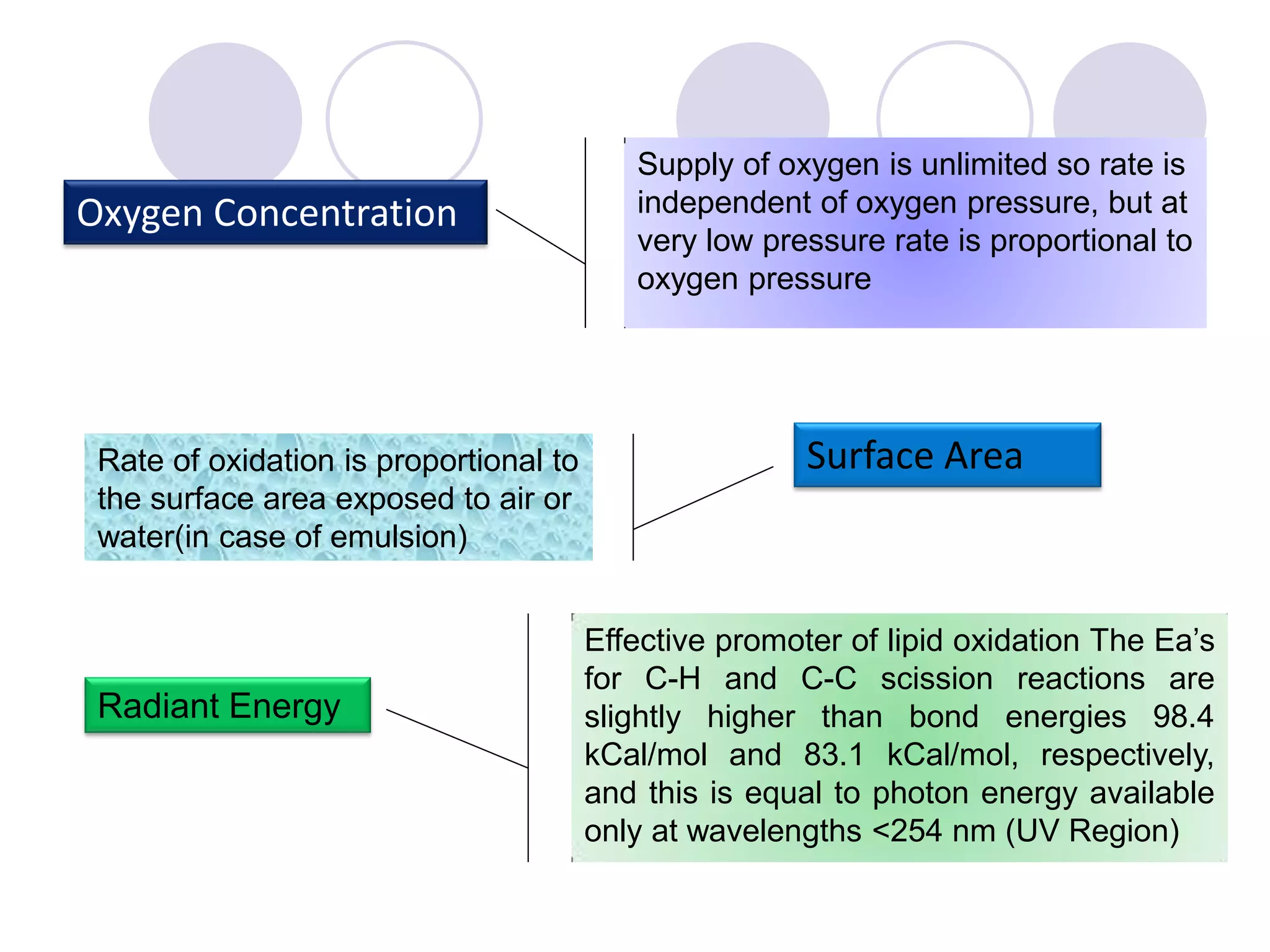
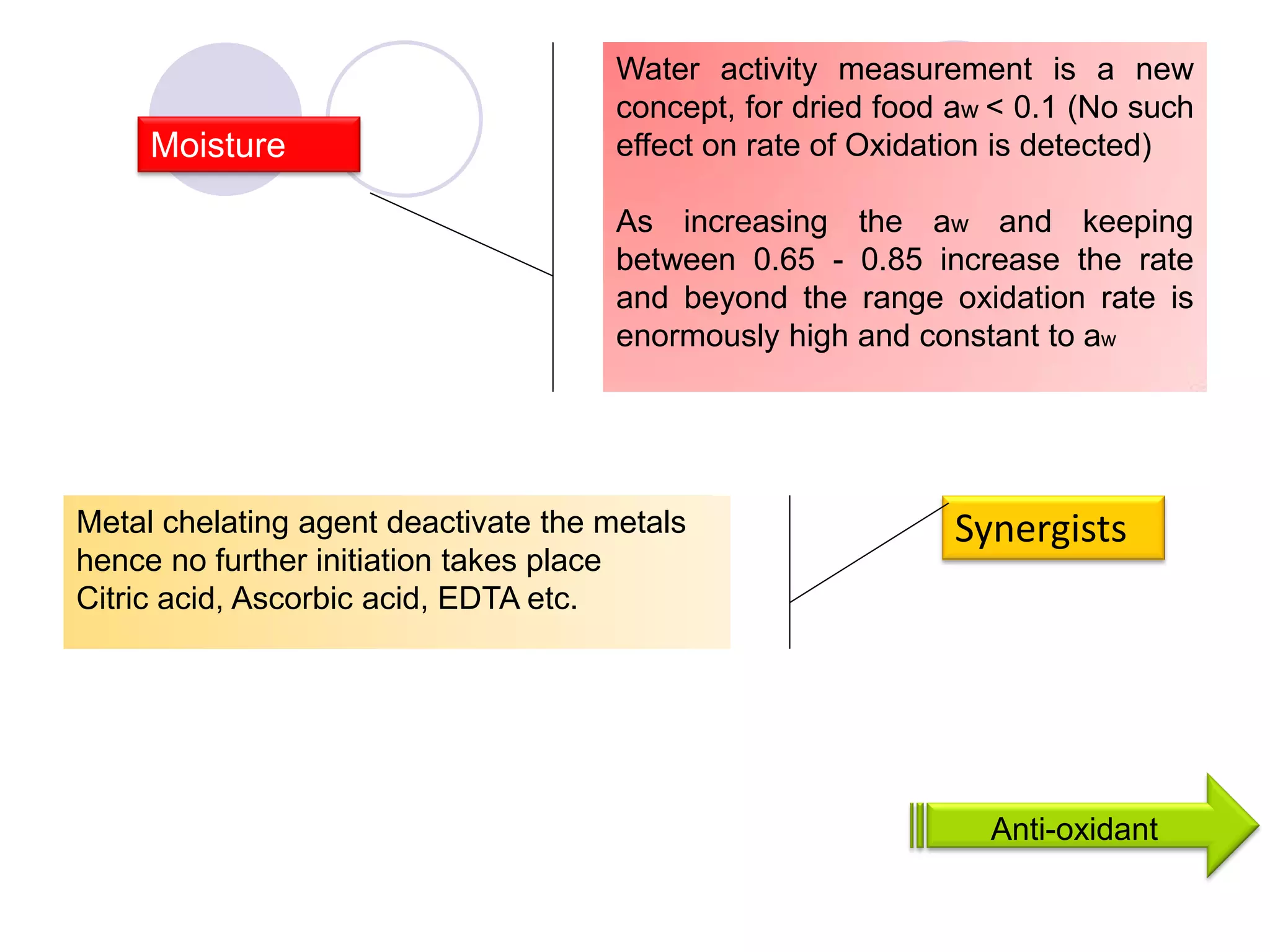
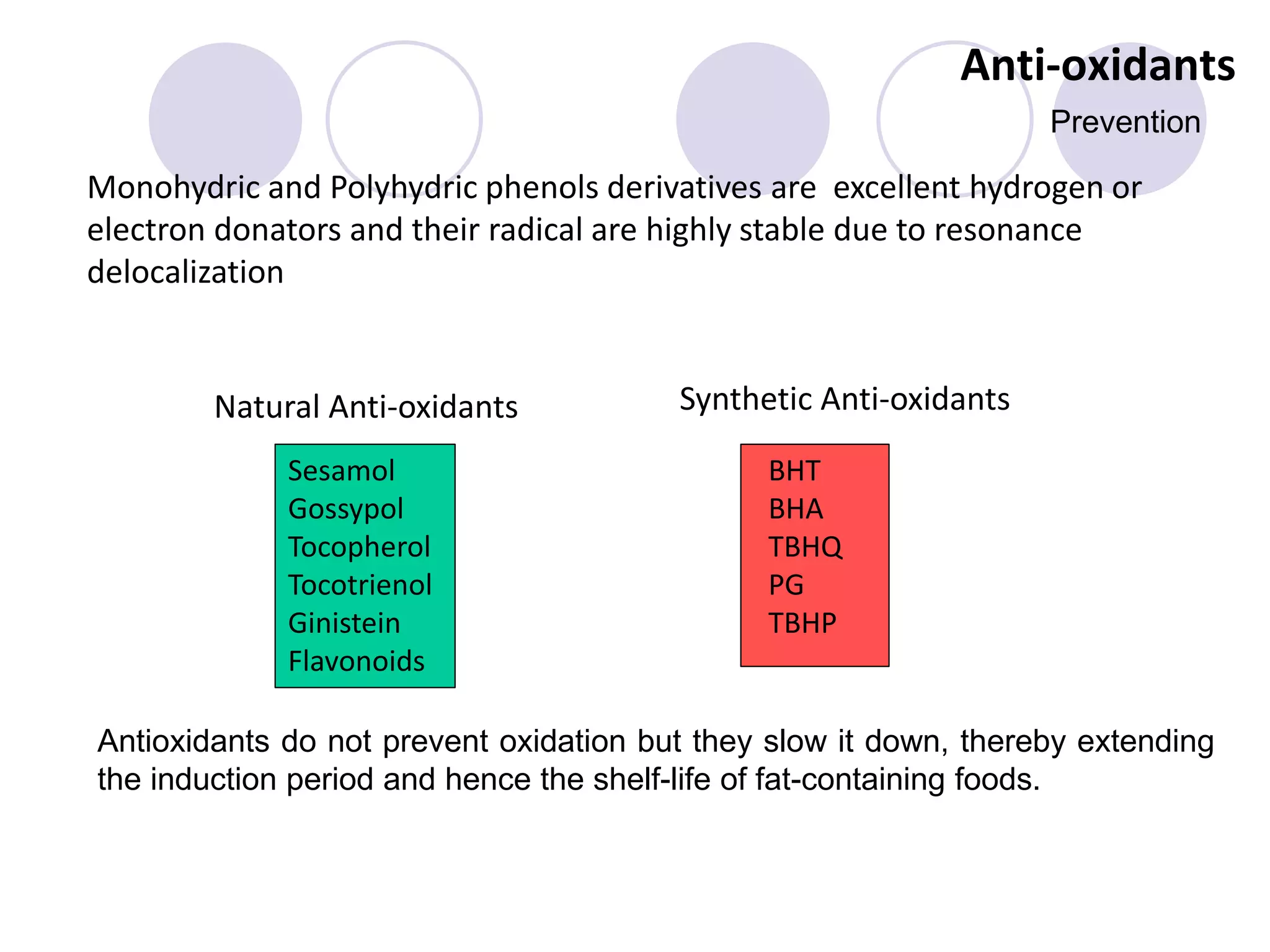
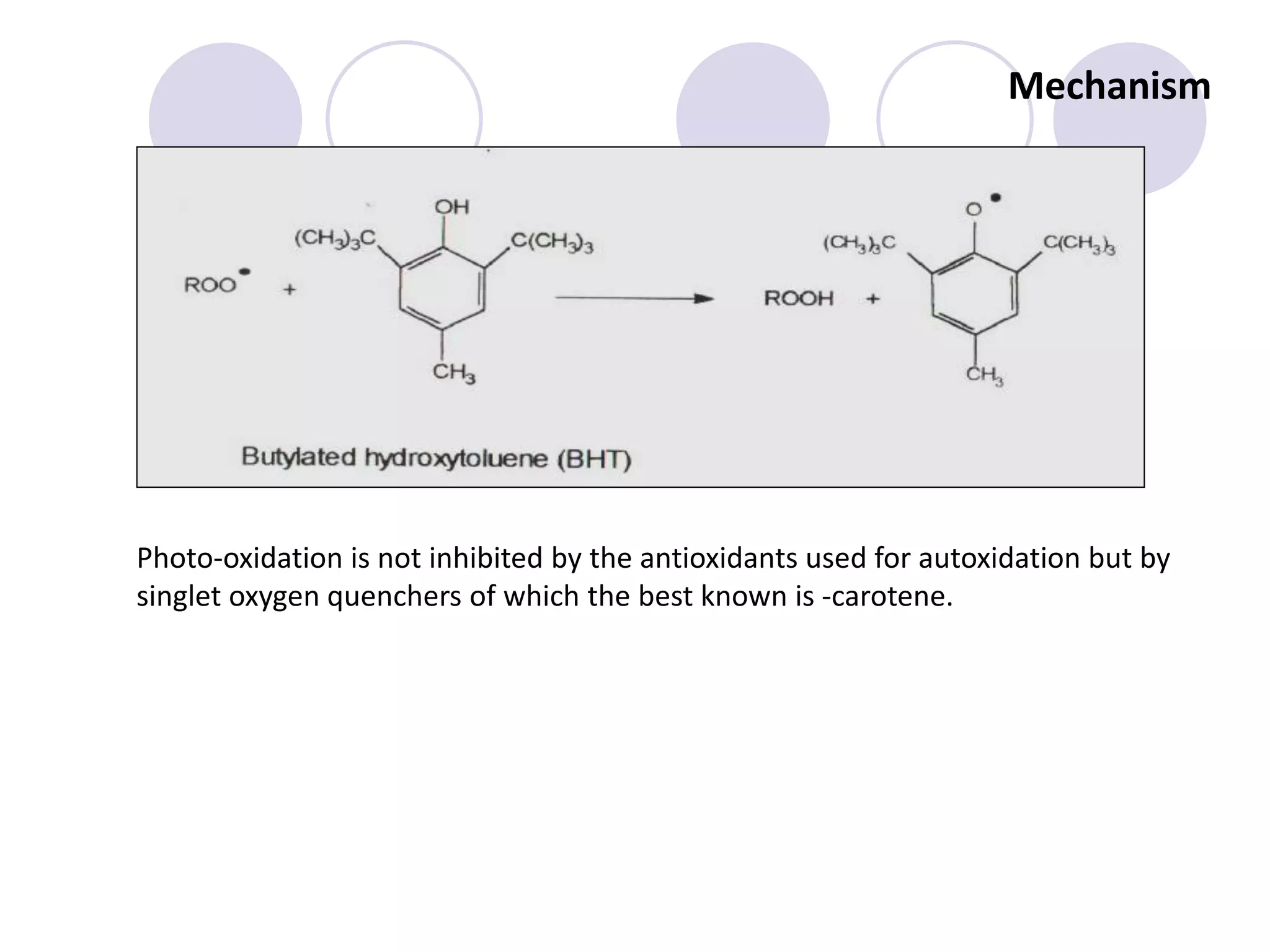
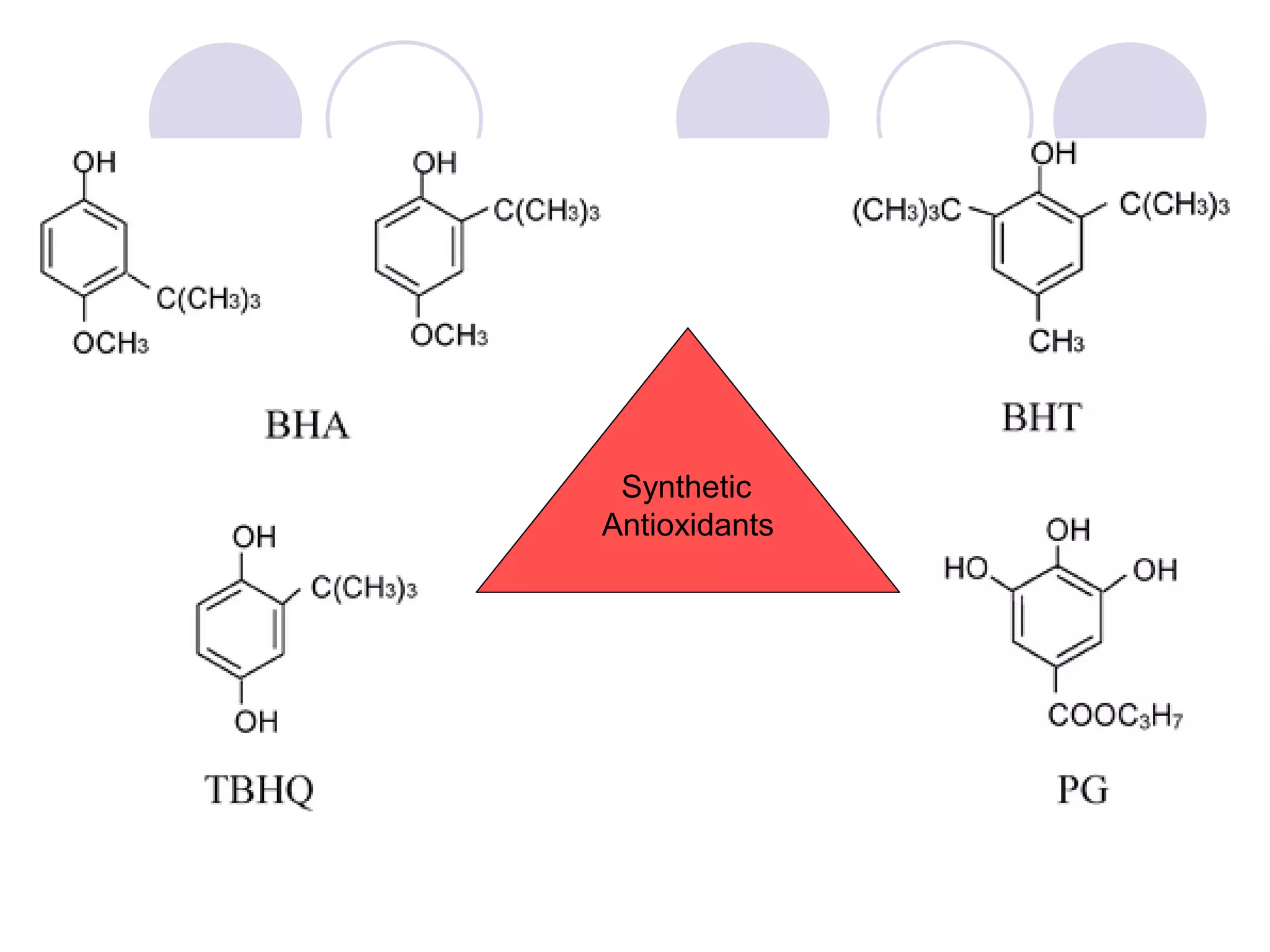
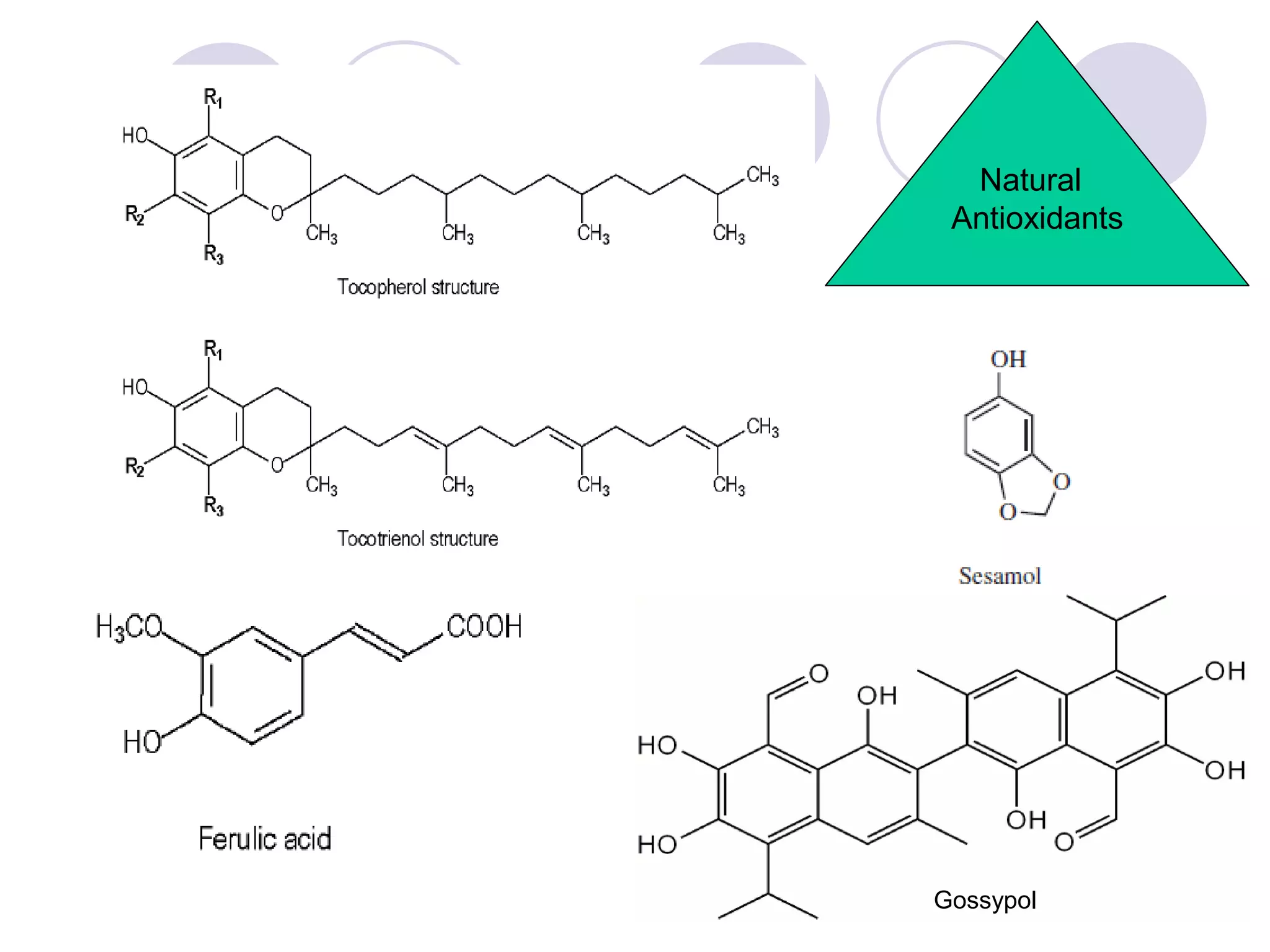
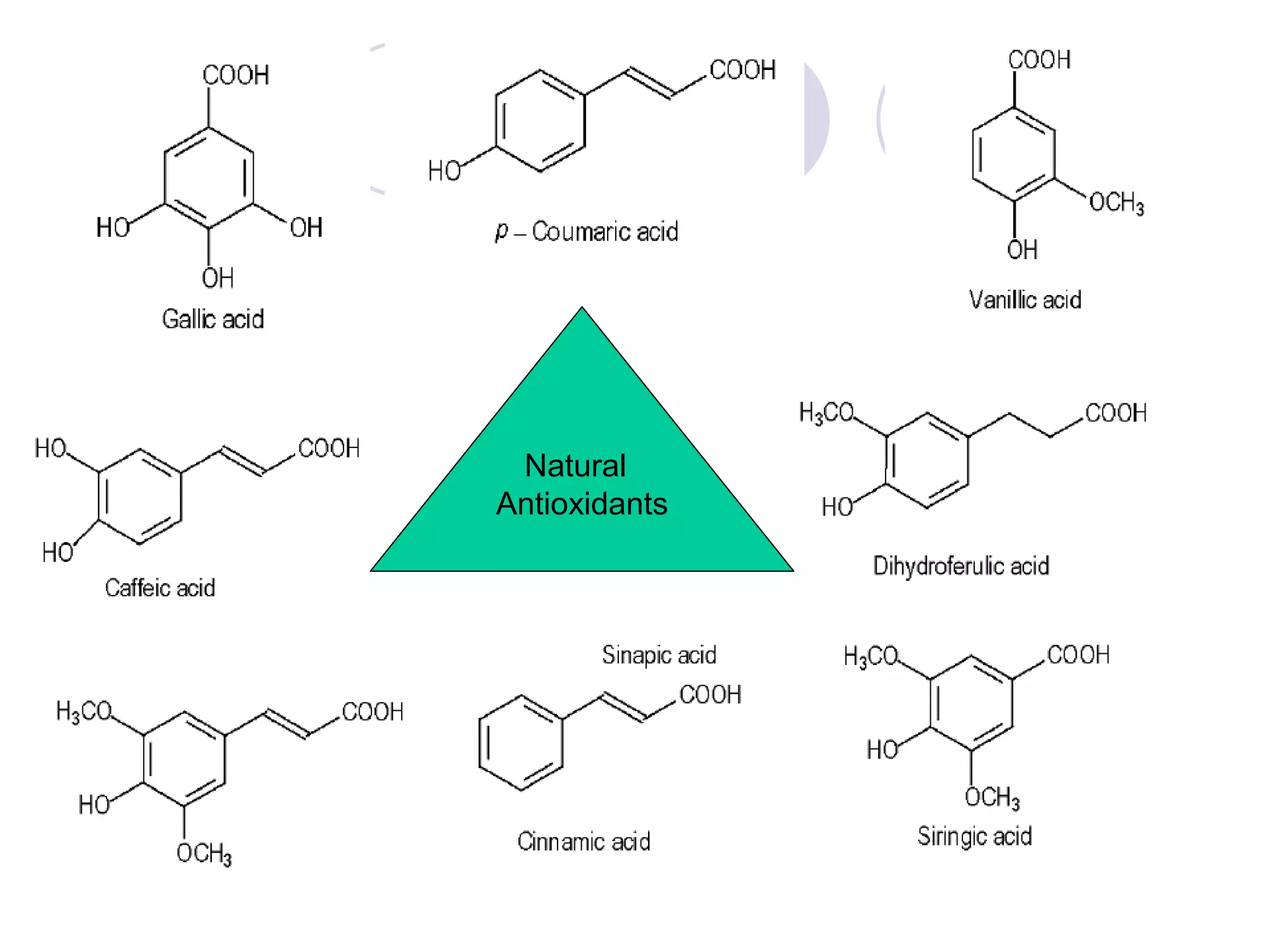
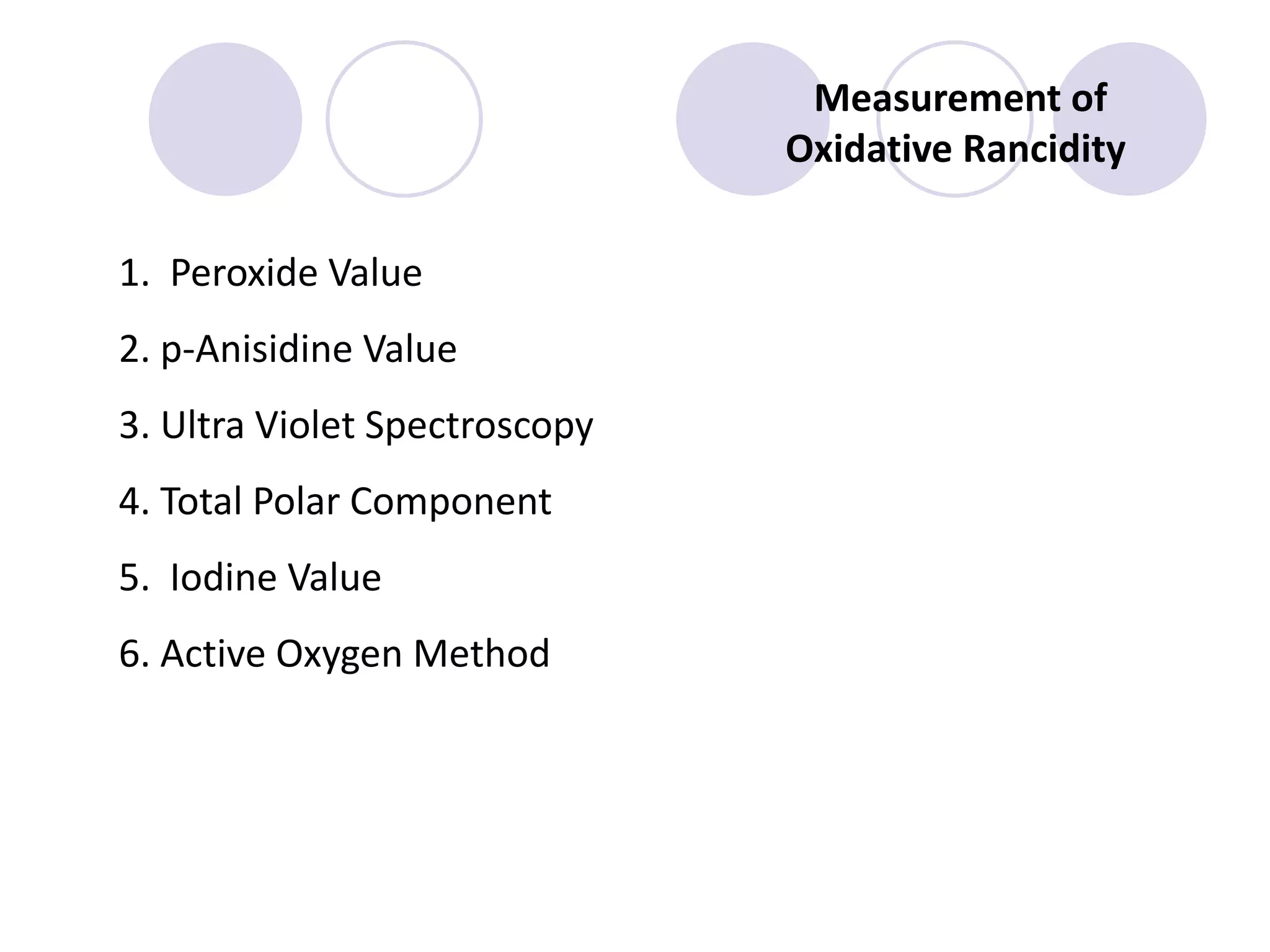
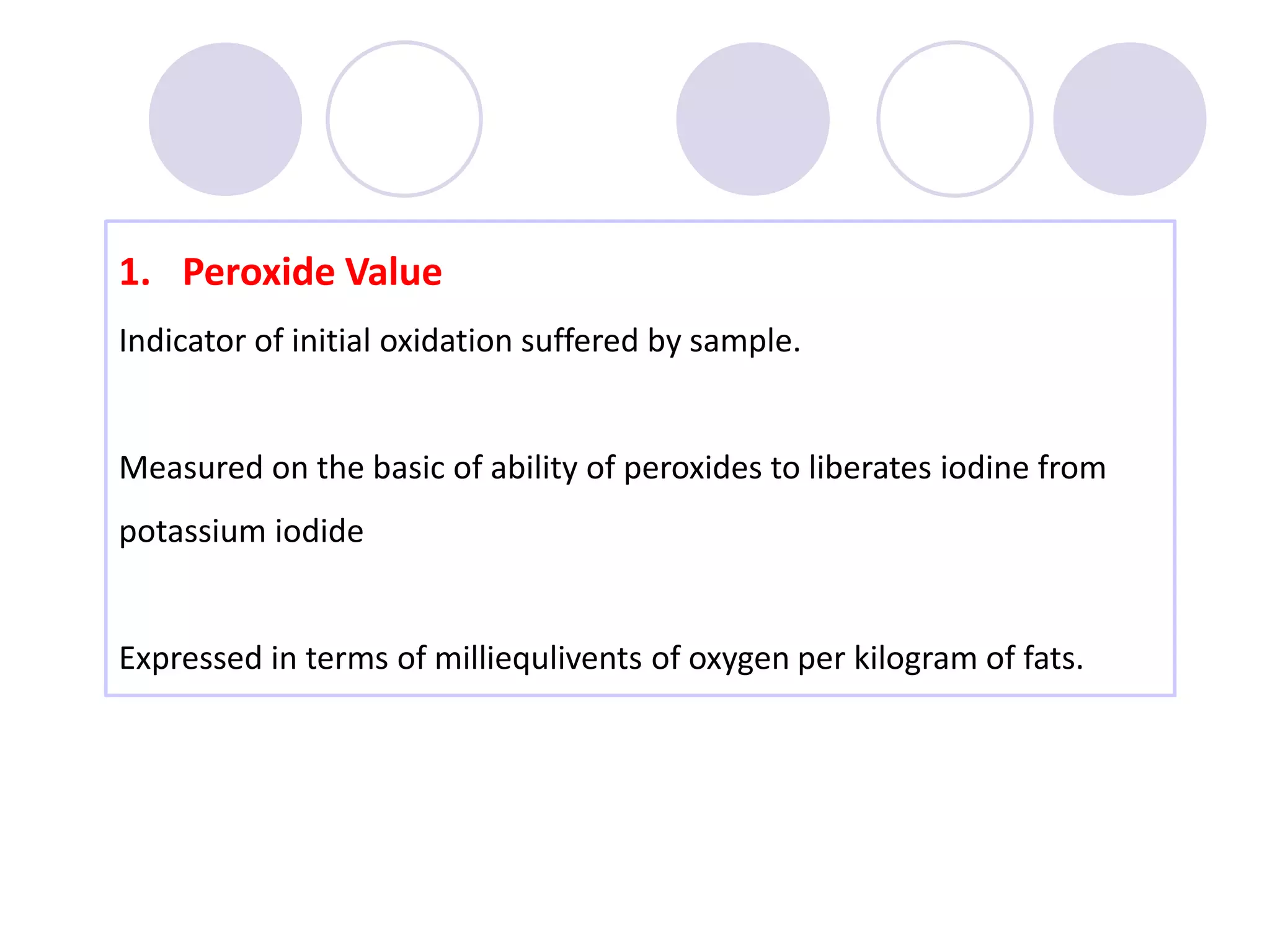
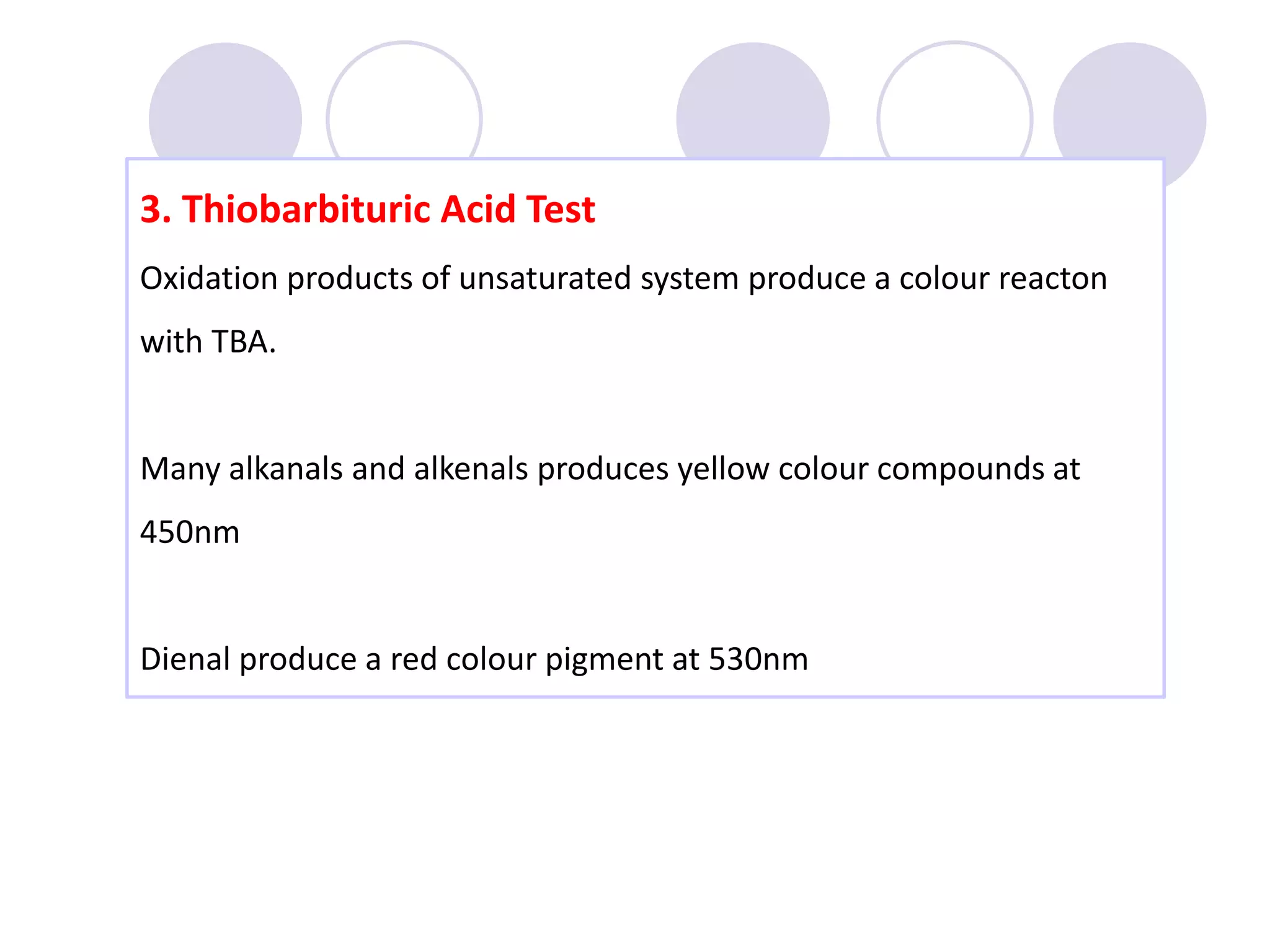
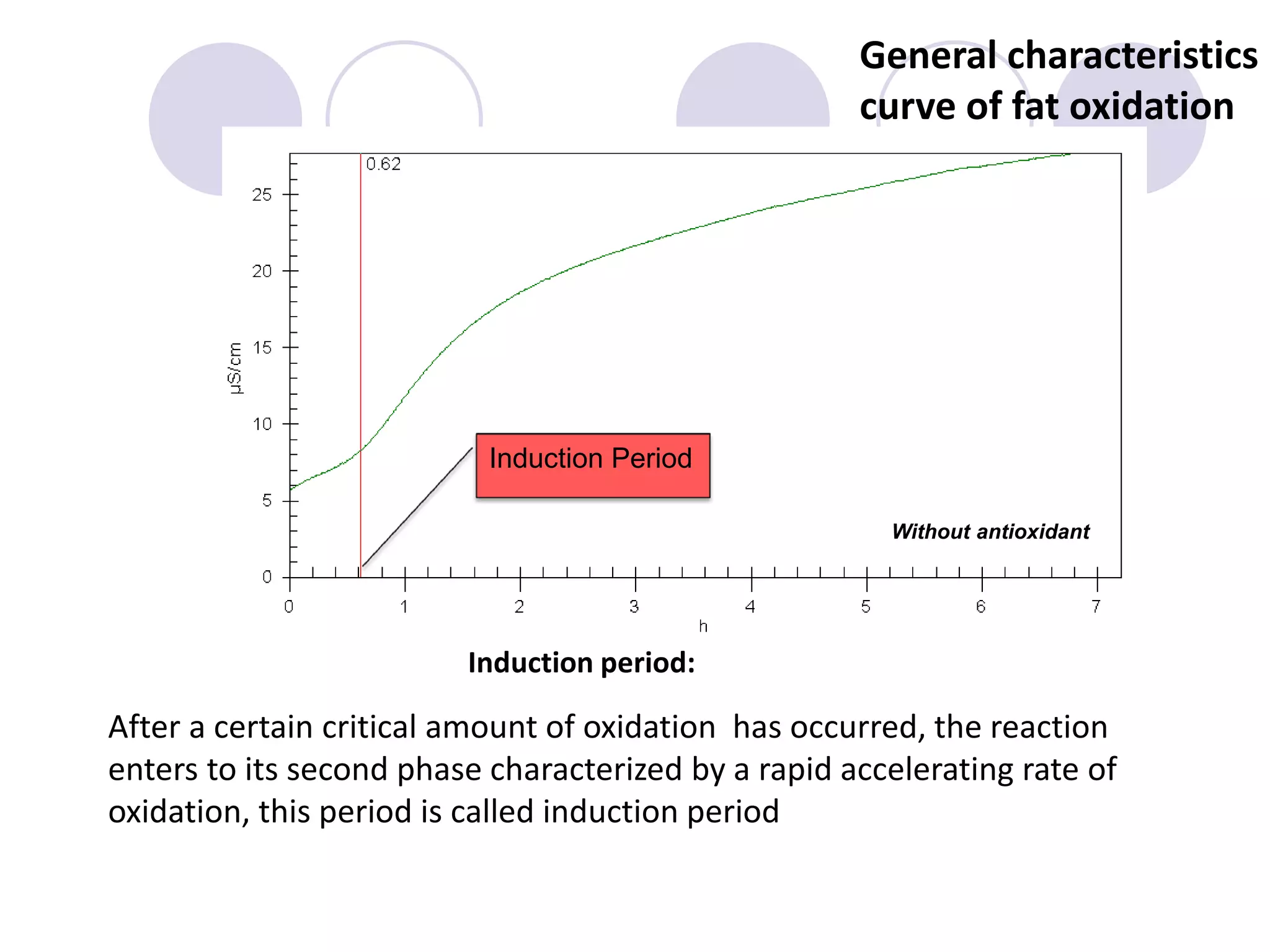
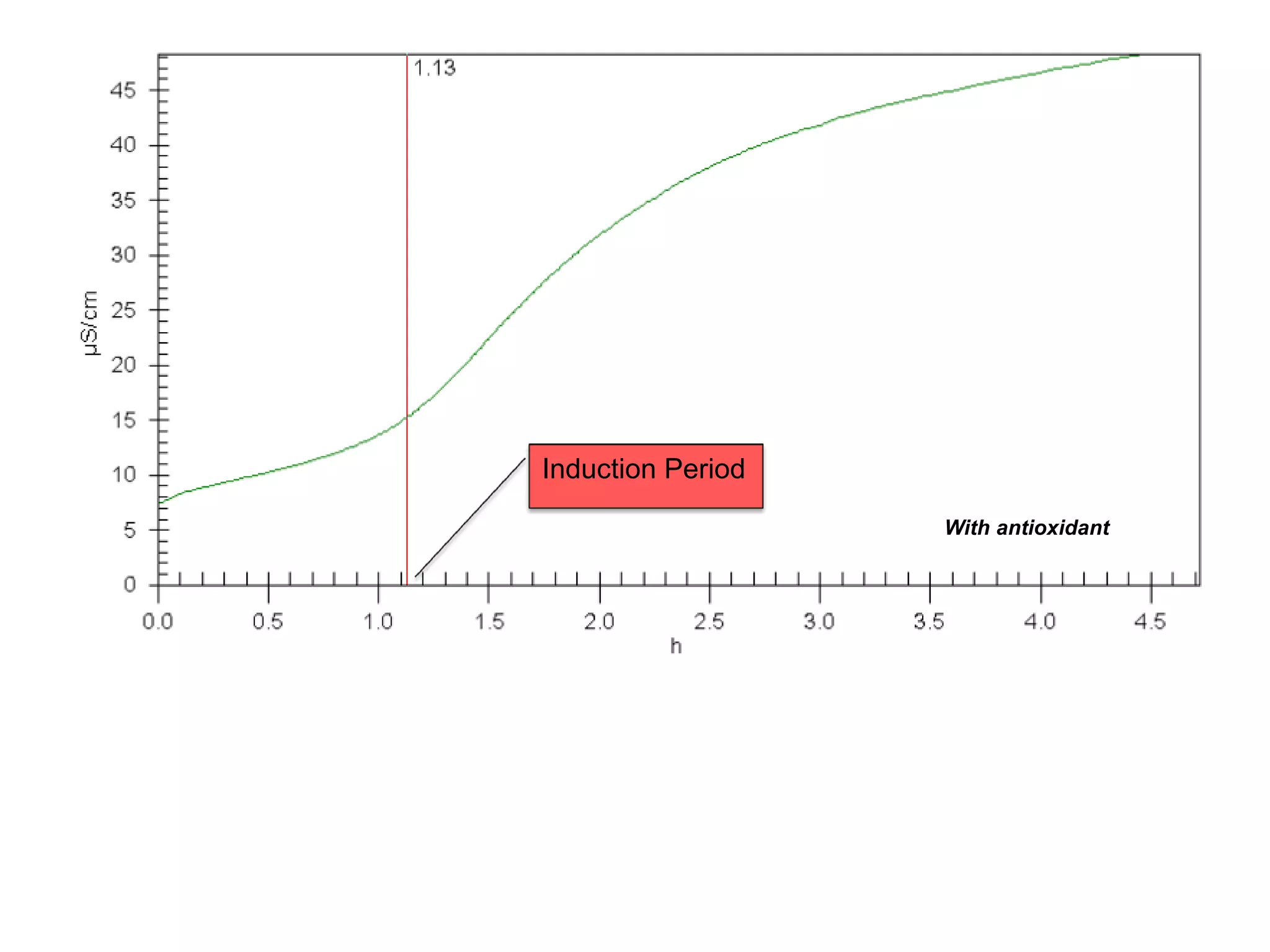
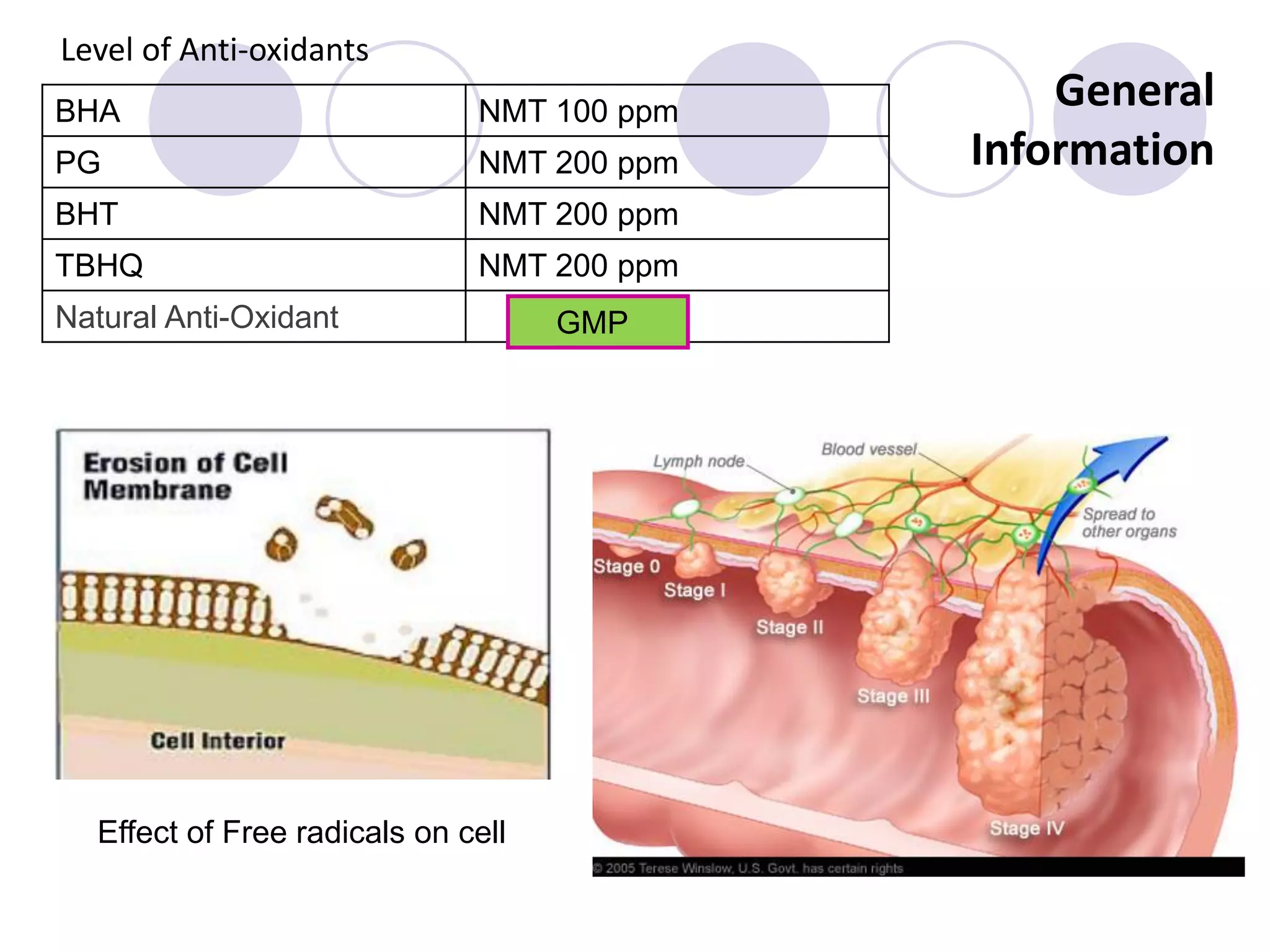
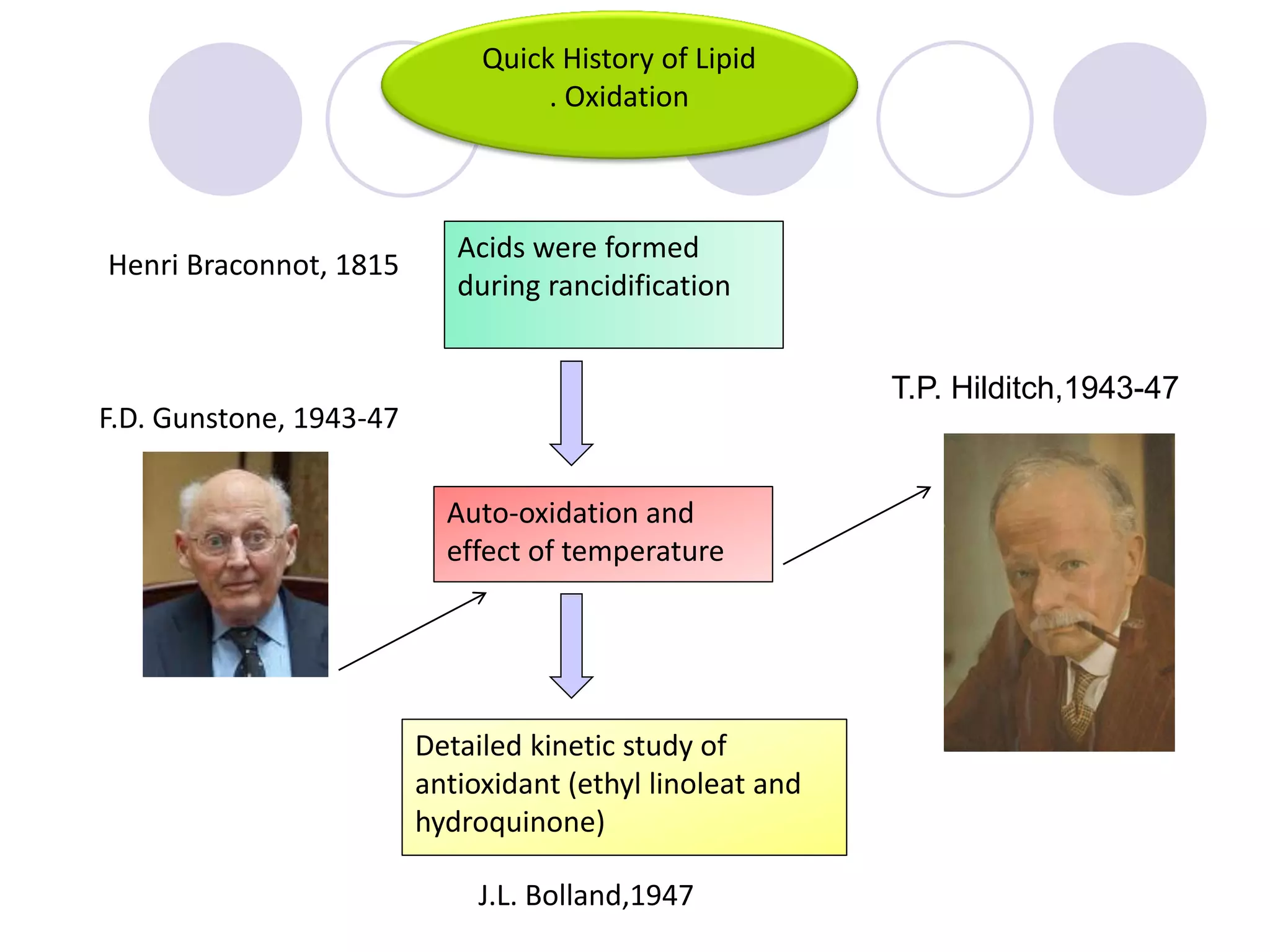
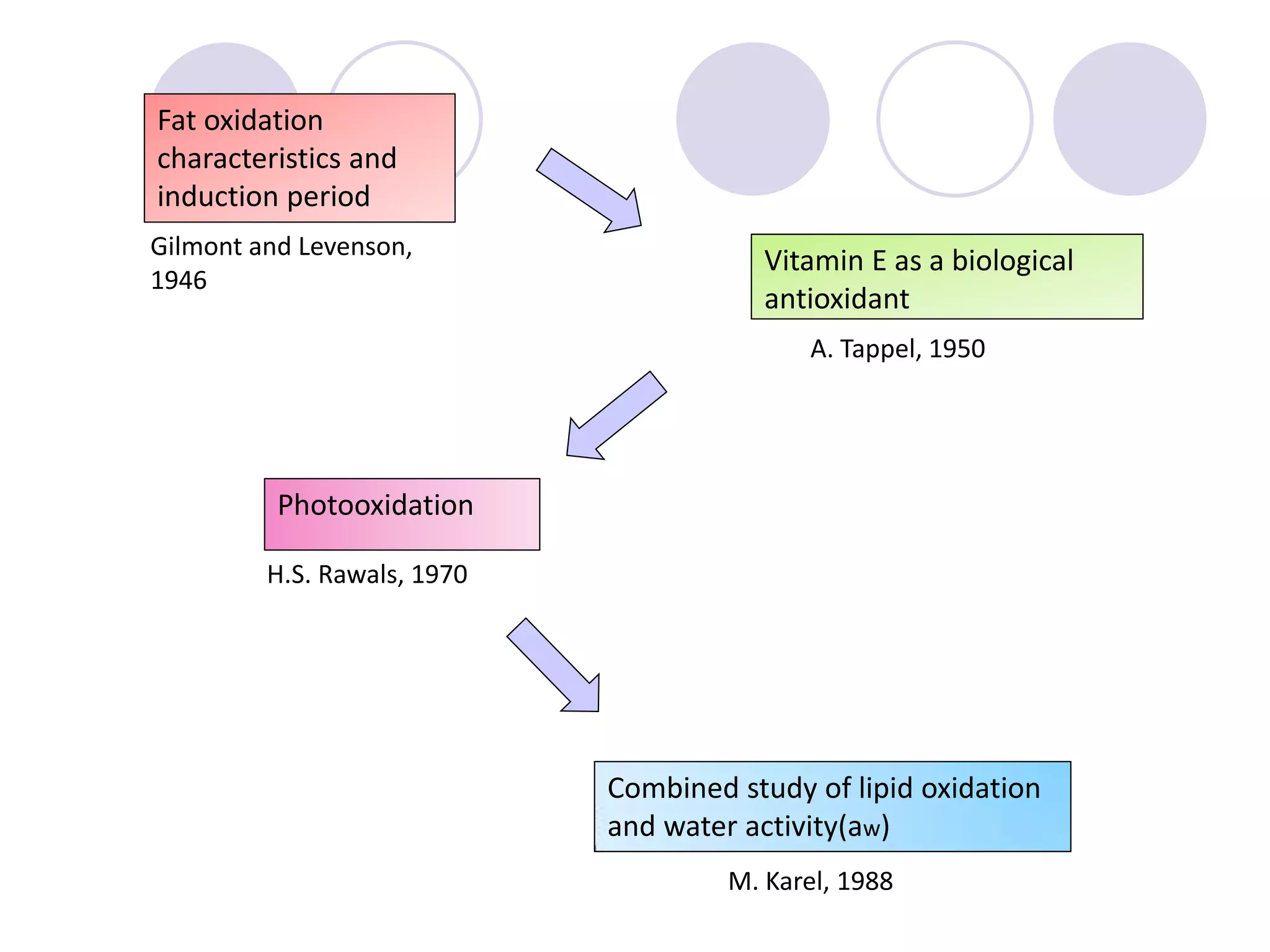
The document discusses lipid oxidation, including types such as hydrolytic and oxidative rancidity, factors affecting these processes, and prevention methods. It highlights the mechanisms of photo-oxidation and auto-oxidation, the role of pro-oxidants and antioxidants, and methods to measure oxidative rancidity. A historical overview of research on lipid oxidation is also provided.