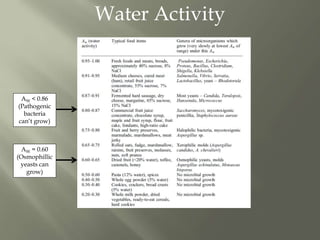
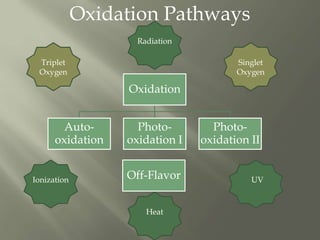
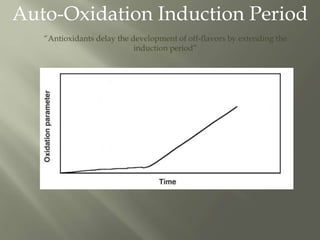
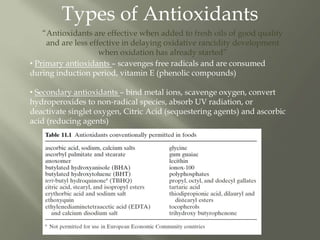
This document discusses shelf life and factors that influence it. Shelf life is defined as the time a food product remains safe, retains desired characteristics, and complies with label declarations when stored under recommended conditions. Factors like raw materials, processing, packaging, storage and consumer handling impact shelf life. Internal changes from moisture, oxygen, light, chemical reactions and microbes also influence shelf life. Packaging acts as a barrier to moisture, gases and light. Microbial growth depends on water activity and relative humidity. Lipid oxidation is a chemical change leading to off flavors and packaging helps prevent photo-oxidation and auto-oxidation through use of antioxidants.