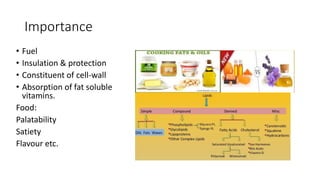
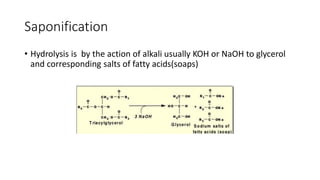
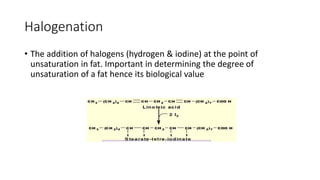
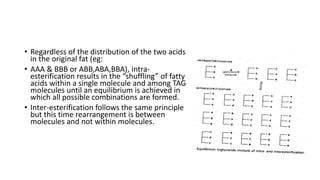
The document discusses lipids, their classifications, properties, and extraction methods. It details the chemical reactions involved in lipid processing, including hydrolysis, saponification, hydrogenation, and inter-esterification, as well as the technologies used in refining and extracting fats. Additionally, it examines the effects of frying on fats, highlighting the significance of oxidative reactions and changes in flavor, quality, and nutritional value of fried foods.