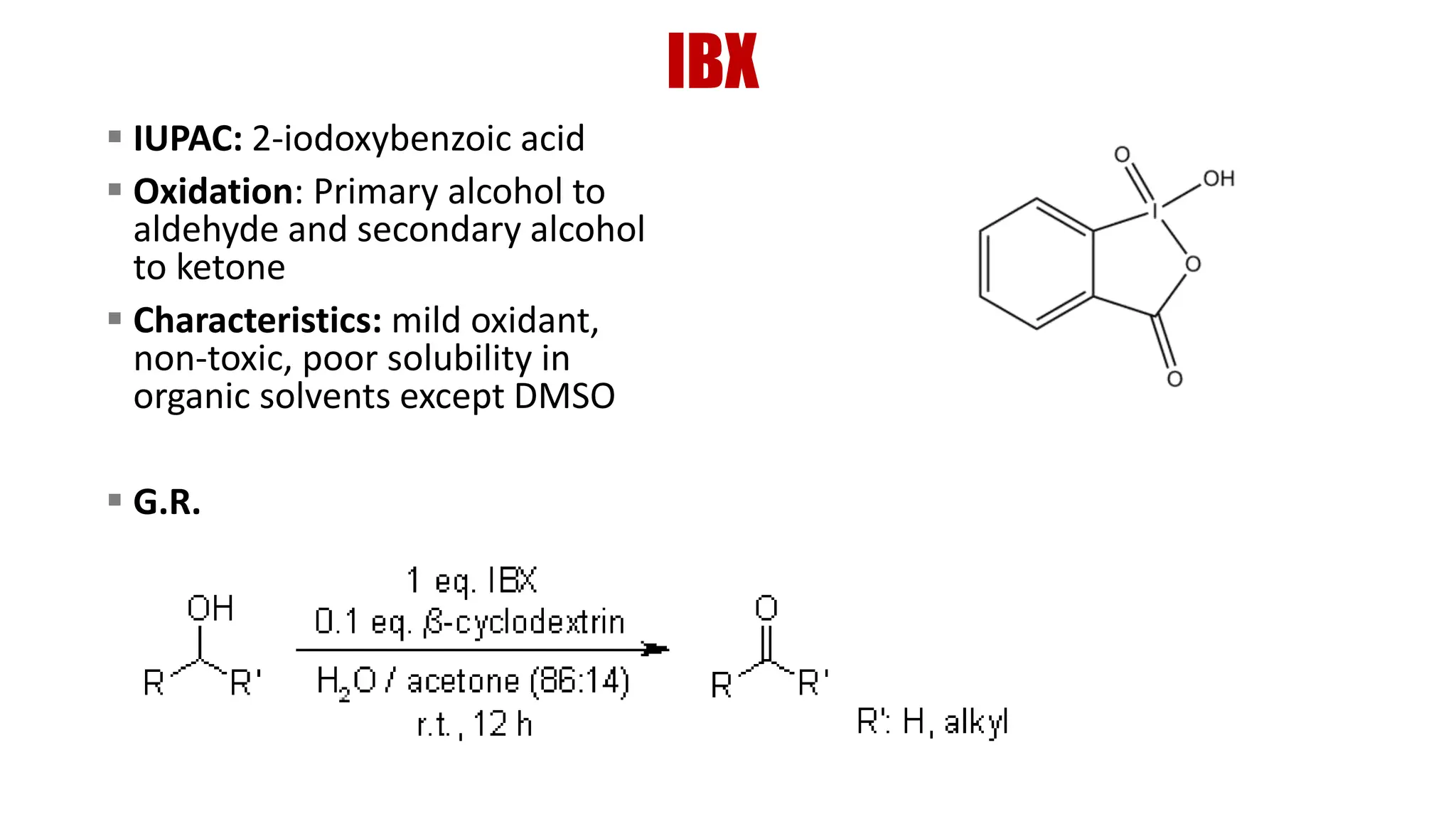
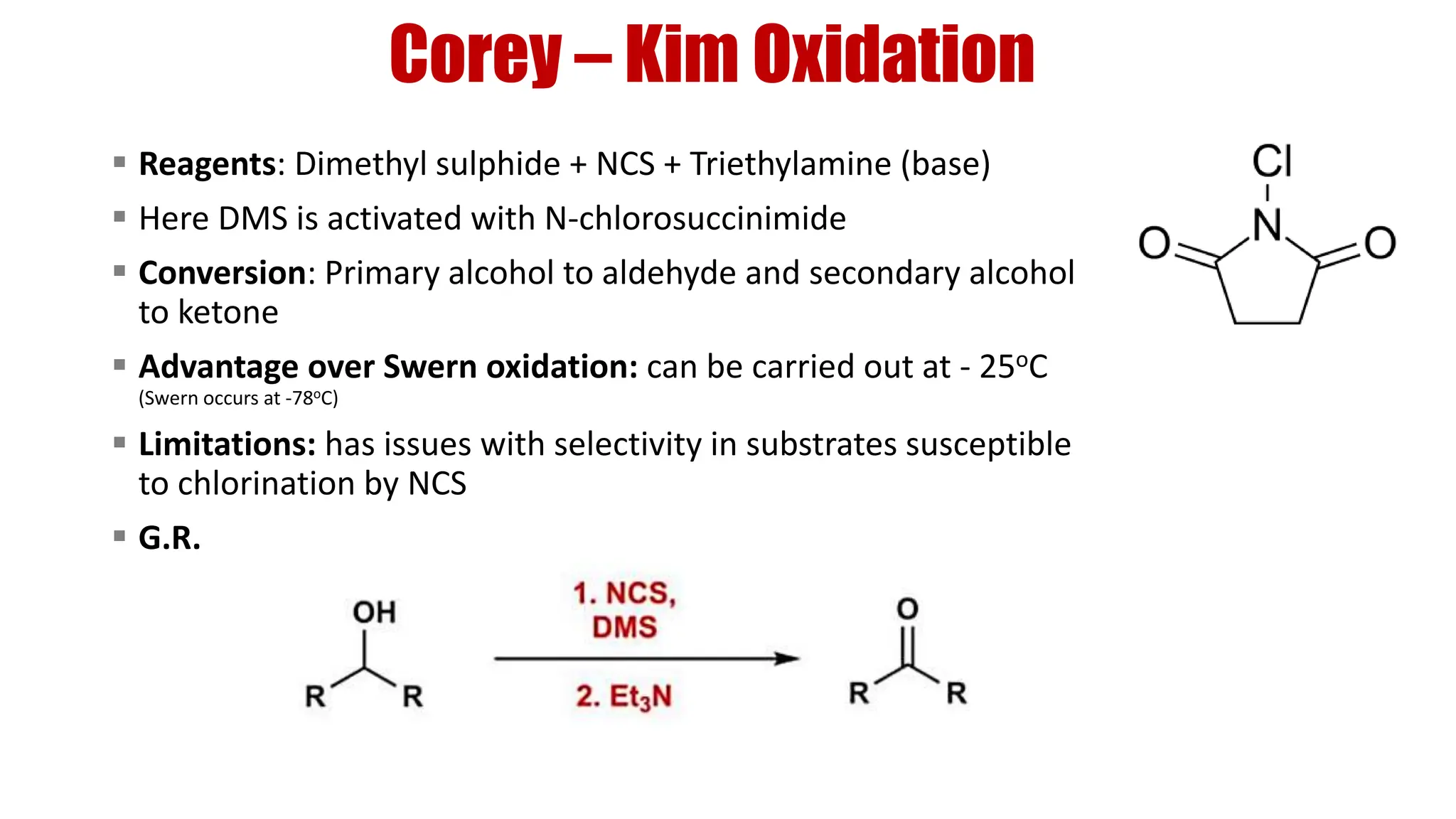
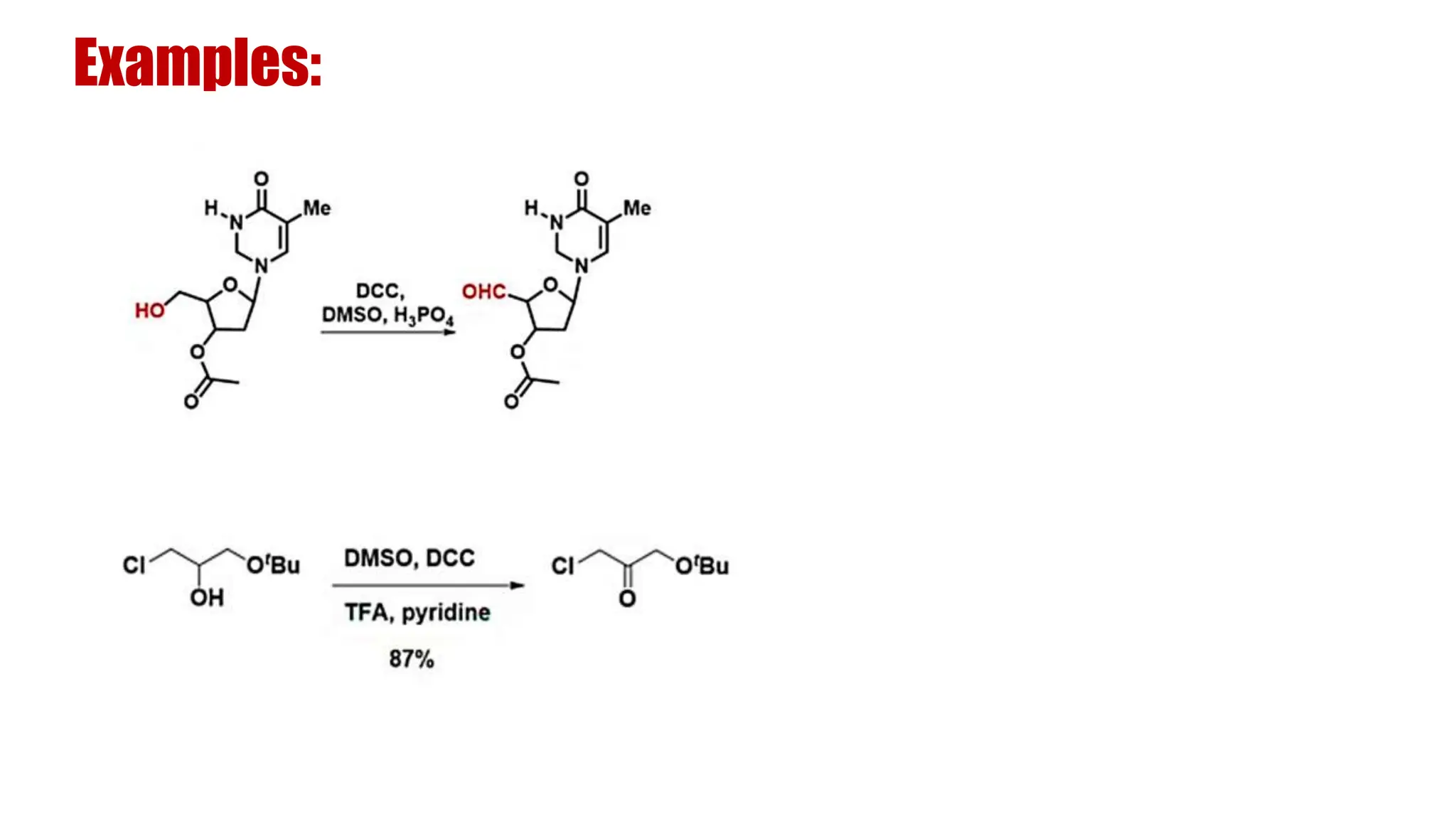
The document discusses the oxidation of alcohols to aldehydes and ketones using various reagents, including hypervalent iodine reagents like Dess-Martin periodinane (DMP) and ibuprofen based reagents. It outlines the general mechanisms, advantages, and limitations of different oxidation methods, such as Swern, Corey-Kim, and Pfitzner-Moffatt oxidations. Additionally, it highlights the use of aluminum isopropoxide in the Oppenauer oxidation for secondary alcohols.