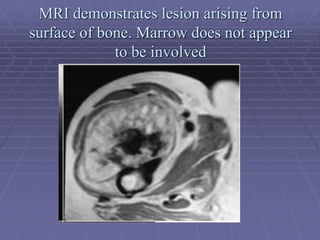
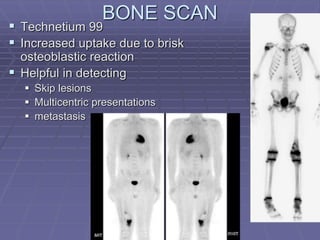
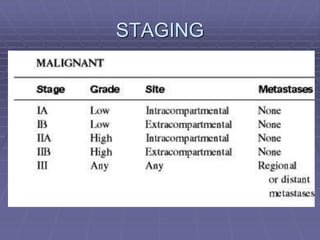
Osteosarcoma is a highly malignant bone tumor characterized by the formation of neoplastic osteoid and bone tissue by malignant mesenchymal cells. It most commonly affects the metaphysis of long bones in children and adolescents. Risk factors include age, radiation exposure, and certain genetic conditions. Patients present with pain and swelling. Diagnosis involves imaging studies and biopsy. Surgical resection is the main treatment, along with chemotherapy. Prognosis depends on tumor grade and stage.