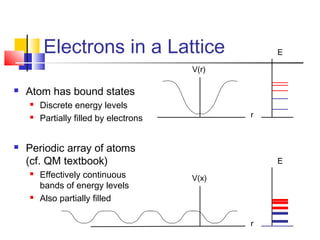
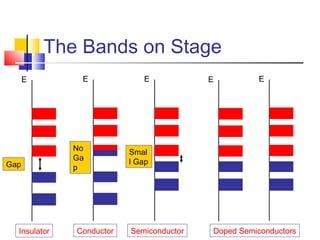
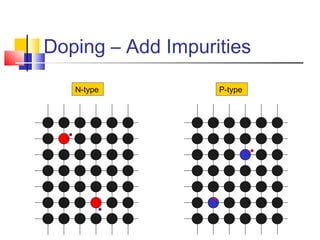
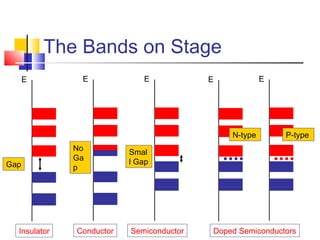
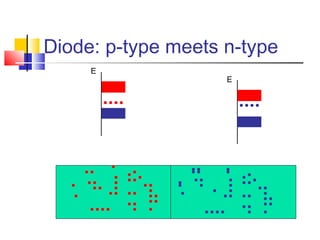
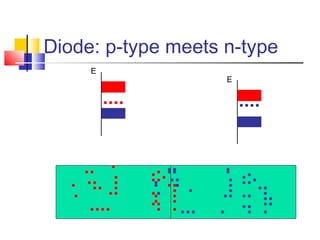
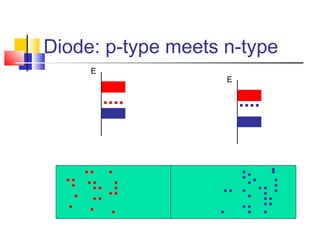
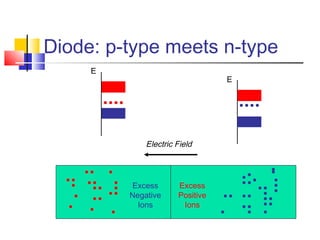
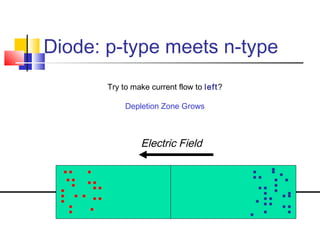
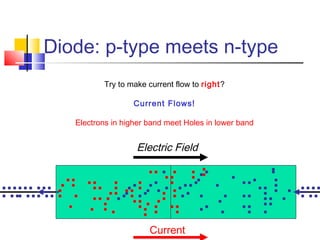
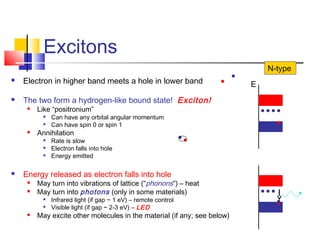
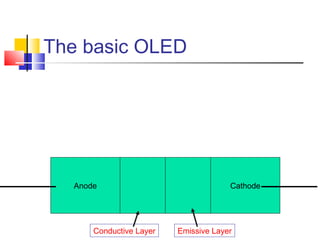
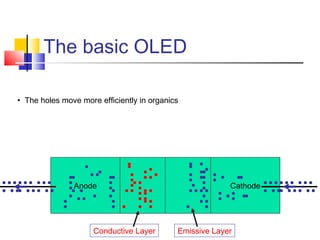
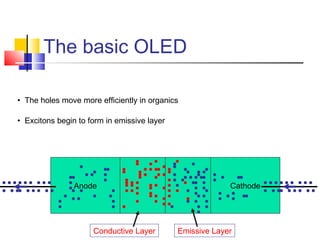
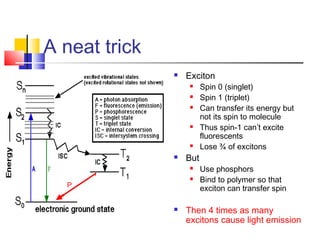
This document discusses organic light emitting diodes (OLEDs) and provides an overview of their advantages compared to traditional lighting and display technologies. OLEDs can provide more efficient lighting, brighter and more versatile displays, and are easier to fabricate than LEDs or LCDs due to their malleable organic materials. The document outlines the basic science of how OLEDs work, including how organic semiconductors transport charges and create excitons that can transfer energy to light-emitting molecules like phosphors to produce light more efficiently than inorganic materials. Overall, OLEDs represent a promising new technology for lighting and displays.