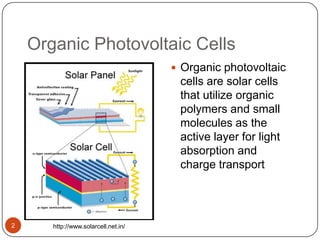
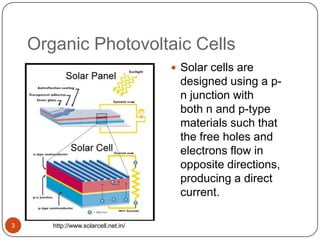
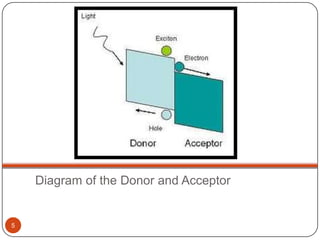
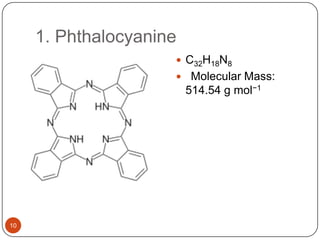
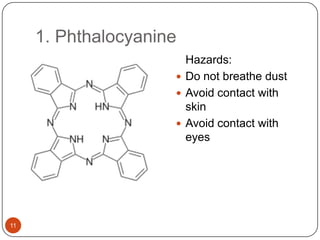
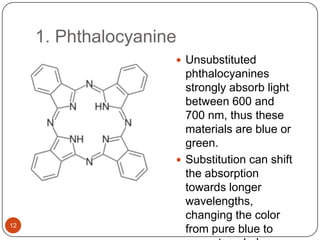
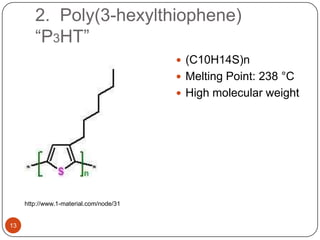
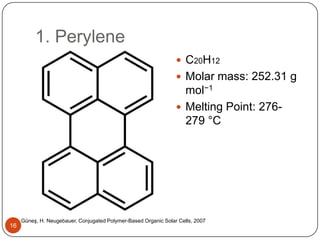
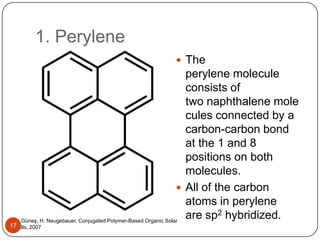
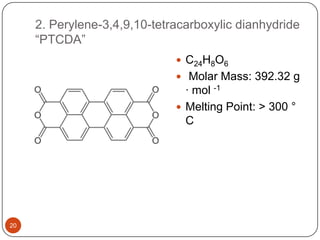
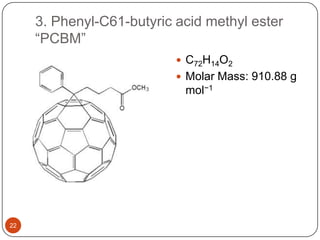
This document discusses polymeric materials used in organic solar cells. It explains that organic solar cells use organic polymers and small molecules to absorb light and transport charges. Common donor polymers mentioned include phthalocyanine and poly(3-hexylthiophene), while acceptor examples provided are perylene, perylene-3,4,9,10-tetracarboxylic dianhydride, phenyl-C61-butyric acid methyl ester, and buckminsterfullerene. The document outlines the charge transfer process in organic solar cells and advantages of using polymeric materials, such as low cost and flexibility. Hazards and properties are also noted for some mentioned materials.