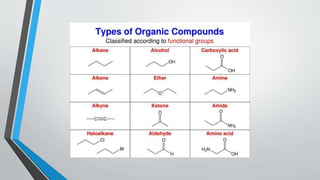
Organic compounds are chemical compounds containing carbon covalently bonded to other elements like hydrogen, oxygen, nitrogen, or halogens. Organic compounds are found widely in nature and living organisms. They have properties like low boiling points, low solubility in water, and high flammability. Organic compounds can be classified based on their source or presence of heteroatoms and include carbohydrates, proteins, alkaloids, fats, oils, hormones, vitamins, and compounds found in natural gas, petroleum, coal, and those synthesized in industries or biotechnology. The structure of organic compounds is based on carbon's ability to form sp, sp2, and sp3 hybrid orbitals and double or triple bonds,