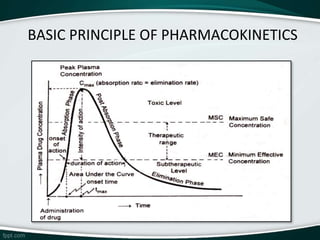
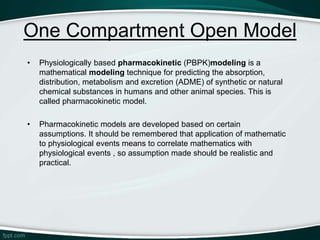
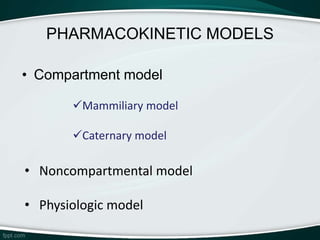
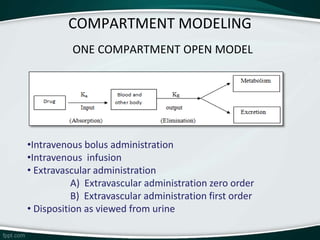
The document provides an overview of pharmacokinetic modeling, specifically one-compartment models used for predicting the absorption, distribution, metabolism, and excretion of substances. It discusses various principles such as first-order and zero-order kinetics, as well as specific modeling techniques like compartmental and physiologic models. Additionally, it includes references to literature and online resources relevant to pharmacokinetics and biopharmaceutics.












![References
• Ali j.et al (2004) “Biopharmaceutics and Pharmacokinetics” 2nd ed. Birla Publications Pvt. Ltd.
Delhi, 64-115
• Analysis of blood & urine data, Compartment models, kinetics of one & two compartment
models [Online] Available at <http://pharmaquest. weebly.com /uploads/9/9/4 /2/994
2916/blood_urine_data.pdf> [Accessed 15-8-2012]
• Brahmankar D.M., Jaiswal S.B. (2009) “Biopharmaceutics and Pharmacokinetics A Treatise”
2nd ed. Vallabh Prakashan, Delhi p.p. 235-298
• Cheng HY, Jusko WJ (1998) Mean residence time concepts for pharmacokinetic systems
with nonlinear drug elimination described by the Michaelis-Menten equation [Online]
Available at <http://www.ncbi.nlm.nih.gov/pubmed/3244627> [Accessed 05-8-2012]
• Compartmental Analysis [Online] available at <http://www.uq.edu.au/ pharmacy/sduf
full/Comp _ analysis.pdf> [Accessed 1-8-2012]
• Dhillon S. and Gill K. Basic pharmacokinetics [Online] Available at <http:// www.
pharmpress.com/files/docs/clinical_pharmacokinetics_samplechapter.pdf>[Accessed 12-8 –
2012]
• Introduction to Pharmacokinetics and Pharmacodynamics [Online] Available at <http://w
ww.ashp.org/DocLibrary/Bookstore/P2418-Chapter1.aspx> [Accessed 12-8-2012]
• Mathematics in Pharmacokinetics What and Why (A second attempt to make it clearer)
[Online] Available at<http://www.cop.ufl.edu/wp-content/uploads/2010/10/PKMATH
stark.pdf> [Accessed 24-8-2012]](https://image.slidesharecdn.com/onecompartmentmodelling-190504052855/85/One-compartment-modelling-17-320.jpg)
![• Mathematics in Pharmacokinetics What and Why (A second attempt to make it
clearer) [Online] Available at<http://www.cop.ufl.edu/wp-content/uploads/ 2010
/10/PKMATHstark.pdf> [Accessed 24-8-2012]
• Multi-compartment model [Online] Available at <http://en.wikipedia.org/wiki/Multi-com
partment_model> [Accessed 29-8-2012]
• Pharmacokinetic Modeling [Online] Available at <http://www.nap.e du/openboo
k.php? record_ I d=11707&page=29> [Accessed 15-7-2012]
• Pharmacokinetics and Biopharmaceutics (46:138) Lecture tutorial [Online] Available
at <http://www.uiowa.edu/~c046138/tut-noncomp.htm> [Accessed 17-7-2012]
• Shargel L. et al (2004) “Applied Biopharmaceutics and Pharmacokinetics” 5th ed. Mc
• Graw Hill’s, North Carolina
• Tipnis H.P. Nagarsenker M.S. (2003) “Introduction to Biopharmaceutics and
Pharmacokinetics” 2nd ed. 2003 Nirali Prakashan India 61-92
• Venkateswarlu V. (2004) “Biopharmaceutics and Pharmacokinetics” Pharma Book
Syndicate, Hyderabad 170
• What are compartmental models? [Online] Available at<http://blog.learnpkpd. com/
2011/01/05/what-are-compartmental-models/> [Accessed 29-8-2012]
• Available <http://www.sciencedirect.com/science/article/pii/S 0304401705002591&d
o cid=v8E_yvAgSz46FM&imgurl=http://ars.els-cdn.com/content/image/>[Accessed
9-8-2012]](https://image.slidesharecdn.com/onecompartmentmodelling-190504052855/85/One-compartment-modelling-18-320.jpg)