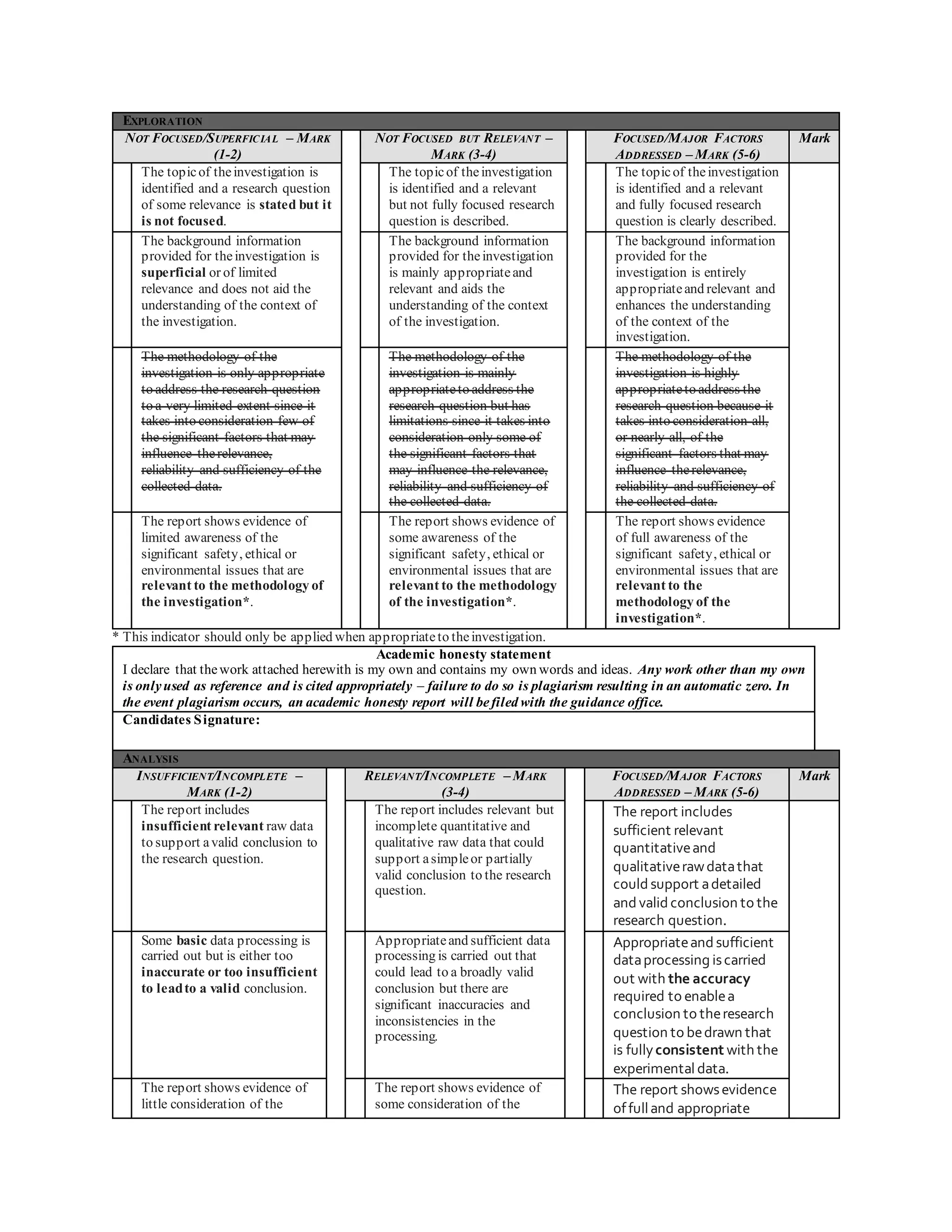
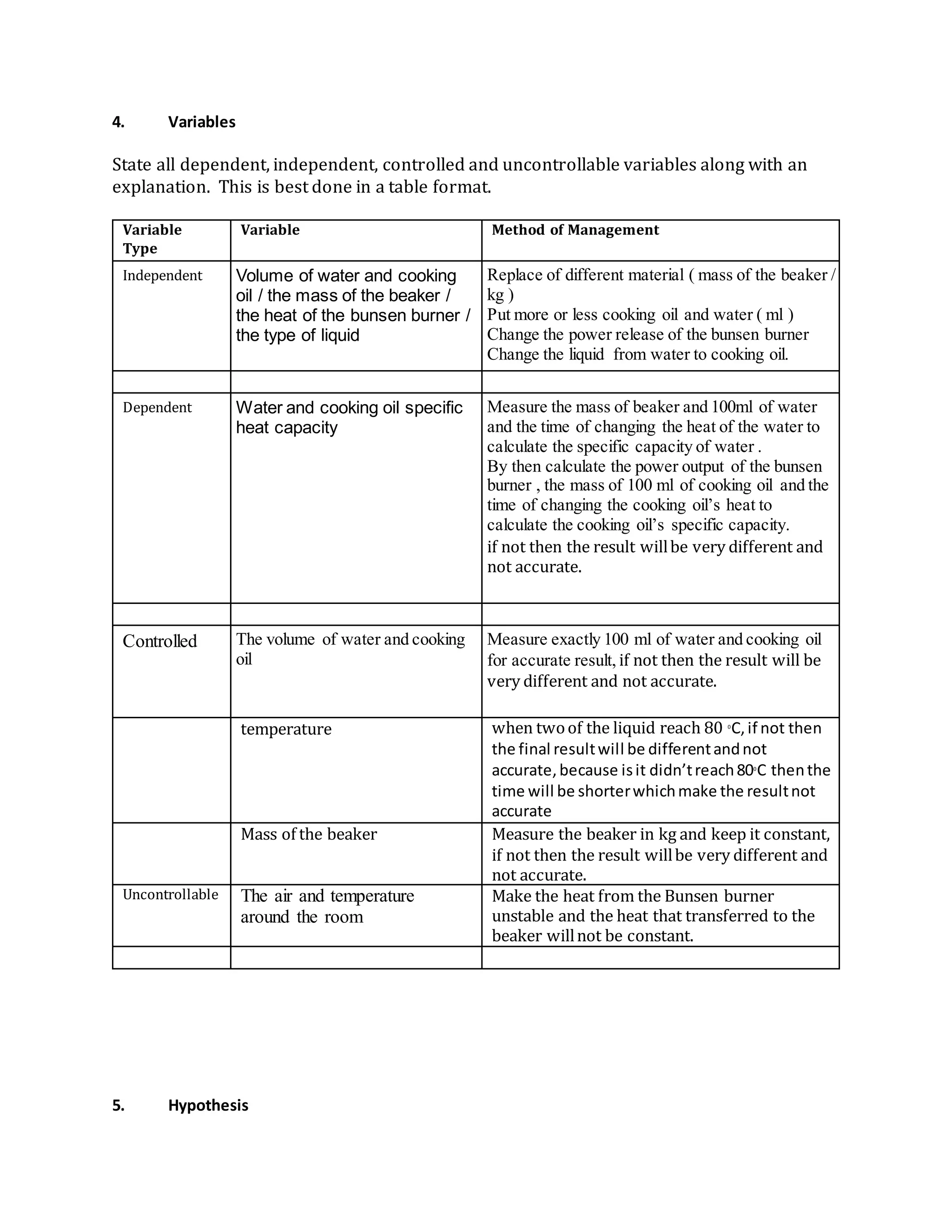
Tam Hieu Nguyen conducted a physics experiment to measure the specific heat capacity of cooking oil. Specific heat capacity is the amount of heat required to raise the temperature of a substance by 1 degree Celsius. Nguyen measured the heat added to a sample of cooking oil and the resulting change in temperature to calculate its specific heat capacity. The experiment was well designed and analyzed, and Nguyen provided suggestions to improve the methodology and extend the investigation further.