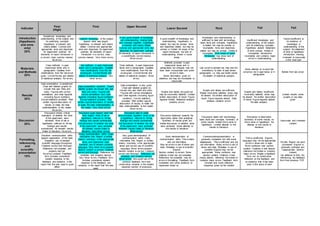
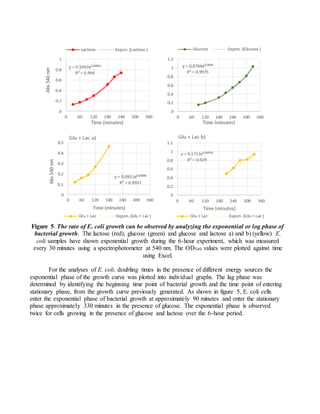
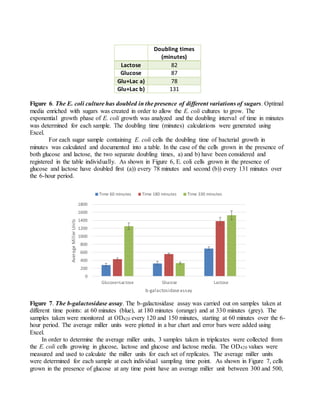
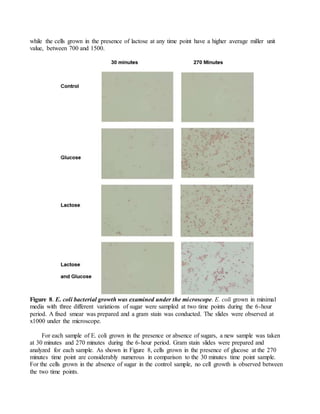
The document provides instructions for completing a formative assessment lab report for a biosciences course. It includes feedback from an assessment of a student's lab report, identifying strengths like detailed diagrams and excellent data analysis, and areas for improvement such as ensuring the introduction is complete. The assessment criteria and levels of achievement for different sections of the lab report are also outlined, including expectations for the introduction, methods, results, discussion, and formatting/presentation. Sample feedback and comments for the student to improve future work are also included.