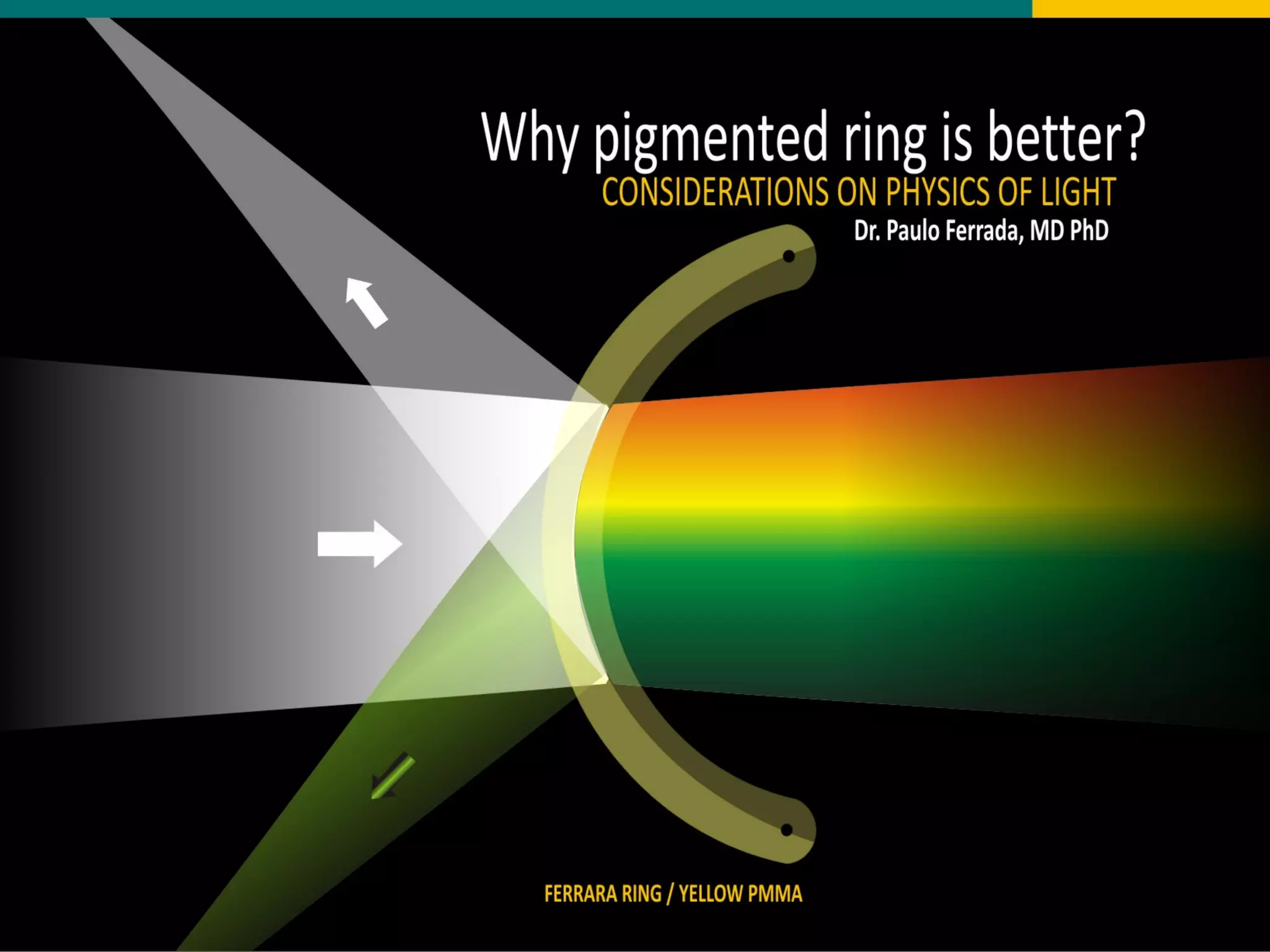
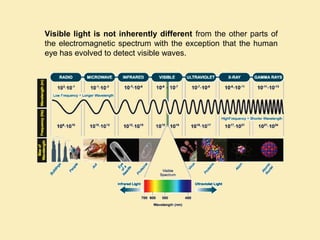
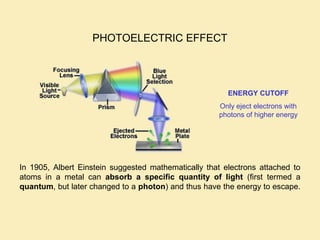
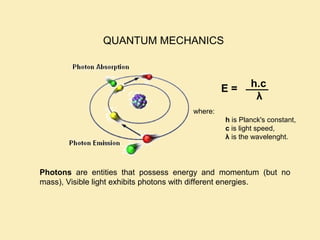
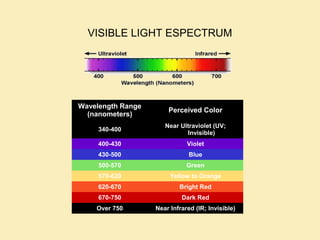
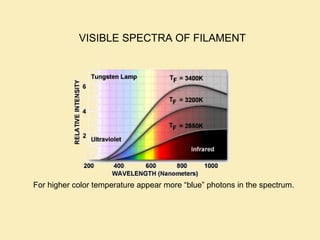
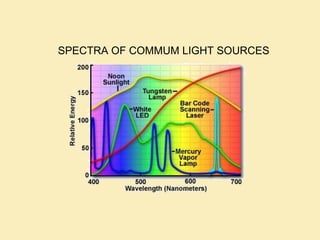
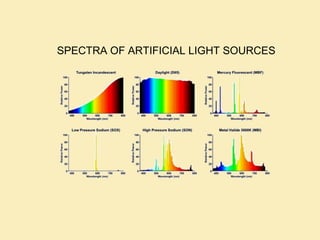
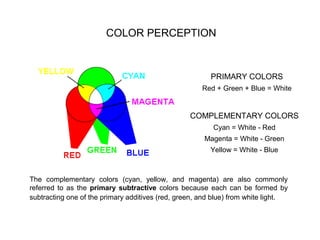
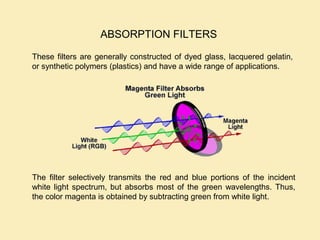
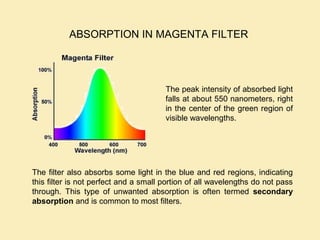
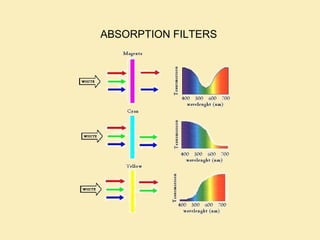
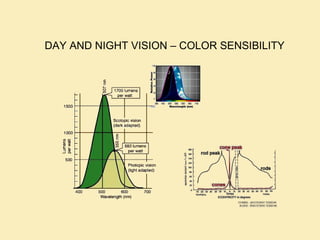
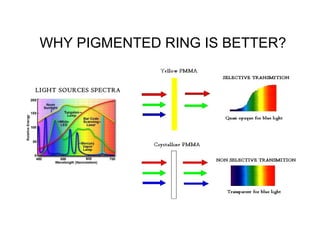
The document discusses the properties of visible light, its interaction with materials, and how humans perceive color. Key concepts include the photoelectric effect, color temperature of light sources, and the role of complementary colors and filters in color perception. Various examples of color temperatures from different light sources are provided, illustrating their impact on color appearance.