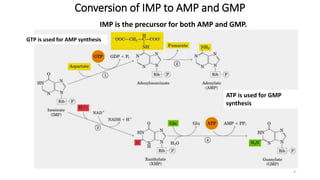
The document discusses nucleotide metabolism and nucleic acids. It provides definitions of nucleic acids, noting they contain carbon, hydrogen, oxygen, nitrogen and phosphorus. There are two types of nucleic acids - DNA and RNA. Nucleotides are synthesized through de novo and salvage pathways. The de novo pathway involves 11 steps to synthesize inosine monophosphate (IMP) from ribose-5-phosphate. IMP is then converted to AMP and GMP. Disorders discussed include gout, which results from high uric acid levels, and Lesch-Nyhan syndrome, a genetic disorder caused by a defect in the HGPRT enzyme involved in purine salvage.





![Regulation of de novo synthesis
• The significance of regulation:
1. Meet the need of the body, without wasting.
2. AMP and GMP control their respective synthesis from IMP by a
feedback mechanism, [GTP]=[ATP]
11](https://image.slidesharecdn.com/nucleotidemetabolism-240312021840-1ba9d98a/85/Nucleotide-Metabolism-biochemistryblecture-pptx-11-320.jpg)
















