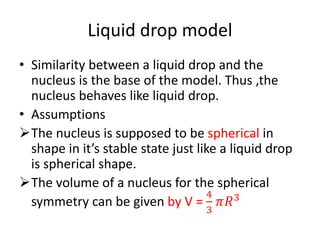
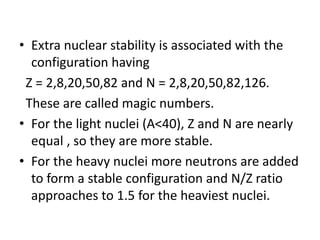
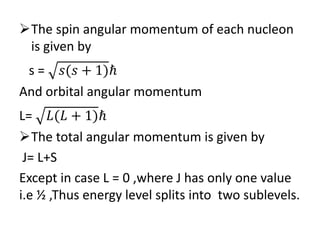
The document discusses three nuclear models: the liquid drop model, Fermi gas model, and shell model. The liquid drop model treats the nucleus like a liquid drop with constant density and assumes nuclear forces are similar to liquid cohesive forces. It can explain nuclear stability, radioactive decay, and fission. However, it cannot explain nuclear spin or magic numbers. The shell model assumes nucleons occupy discrete energy levels or shells in the nucleus similar to electron shells. It explains nuclear properties including magic numbers and stable isotopes. The key assumptions are that nucleons behave independently and occupy shells that align with magic number configurations.