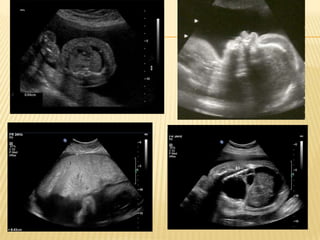
This document discusses nonimmune hydrops fetalis (NIHF), which accounts for 90% of cases of hydrops fetalis. NIHF has numerous potential underlying causes and carries significant risks for both the fetus and mother. The document defines NIHF, discusses its differential diagnosis and evaluation, potential etiologies, presentation, prognosis, management options, and recommendations from clinical practice guidelines.