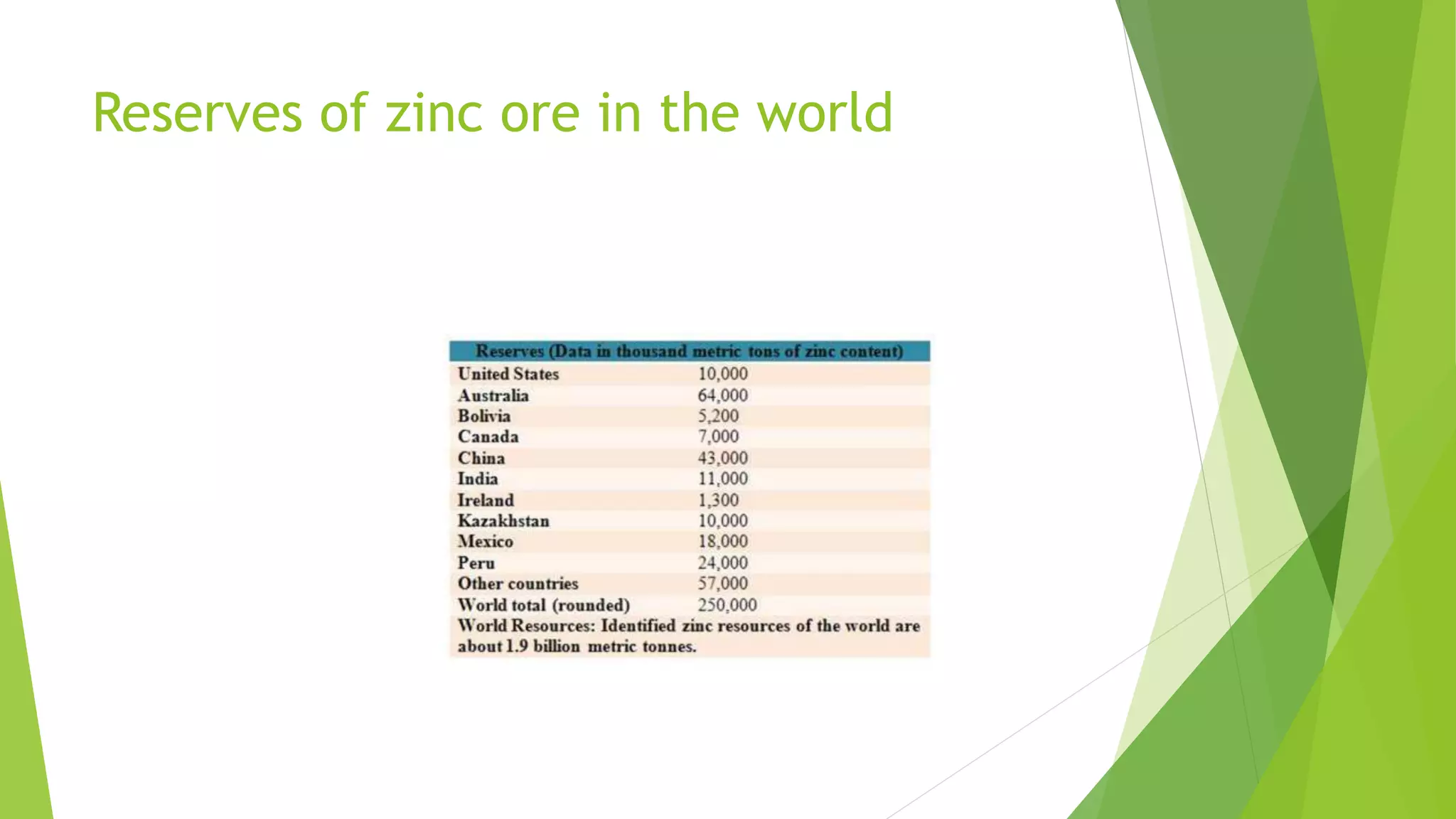
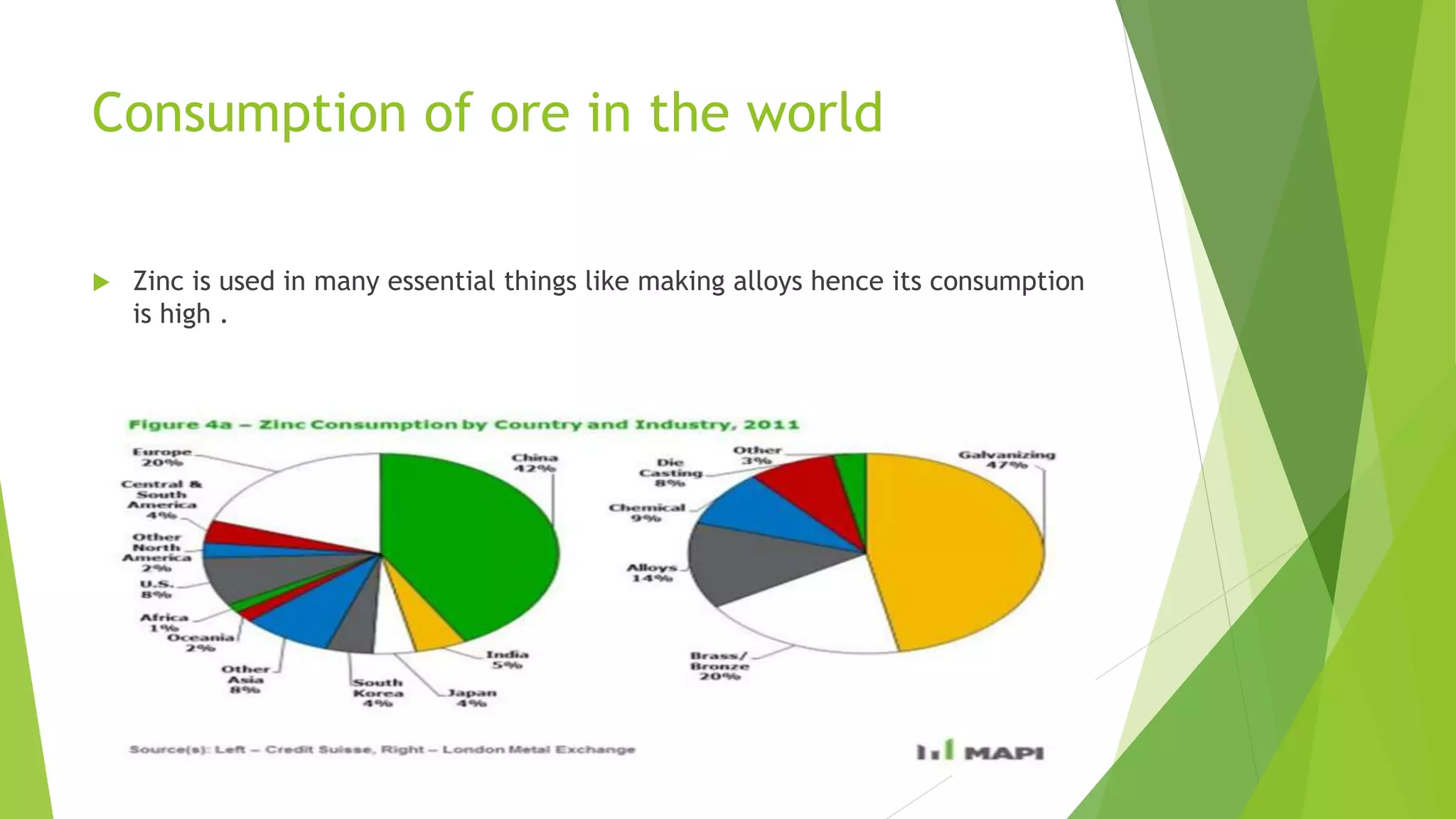
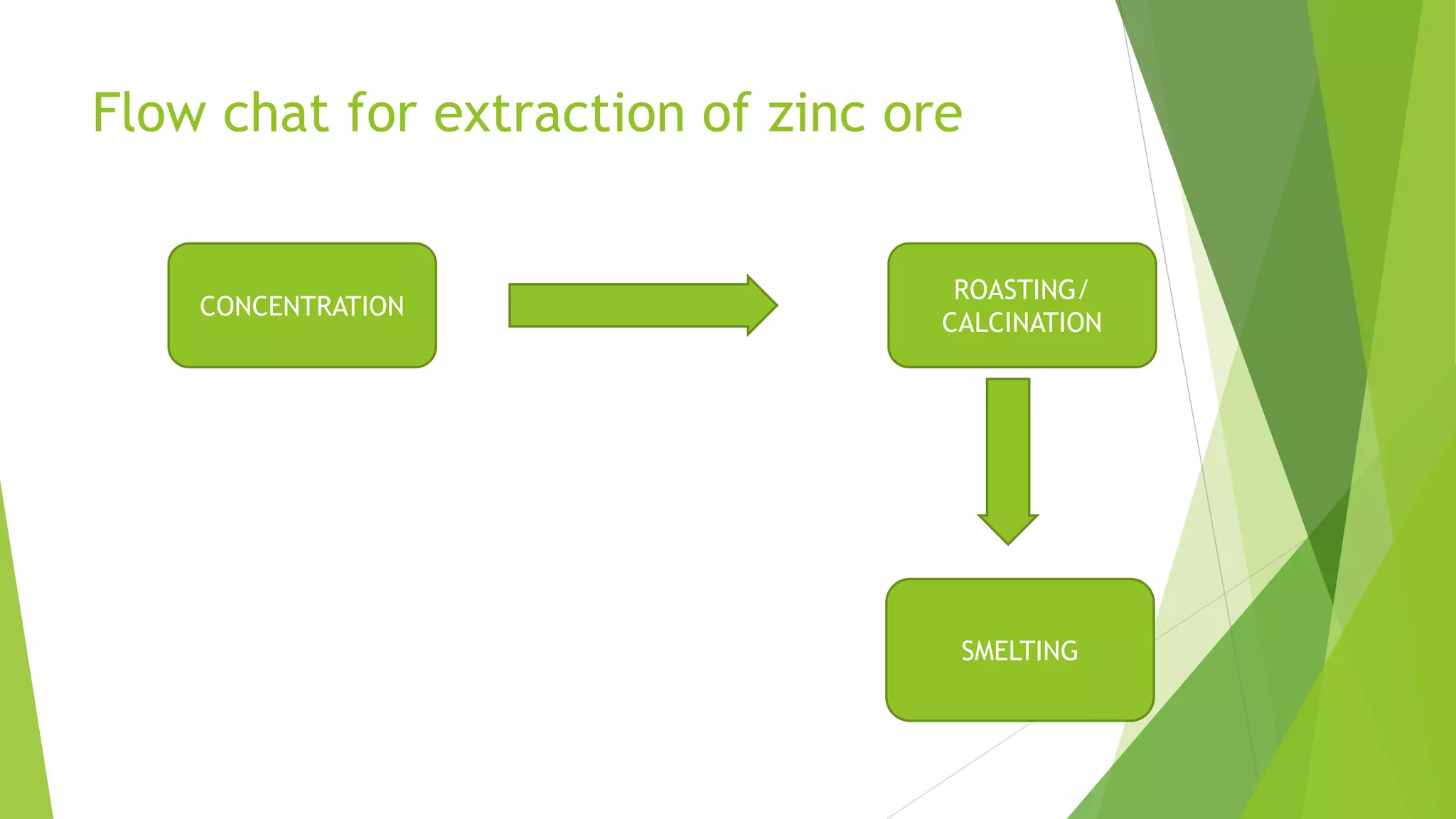
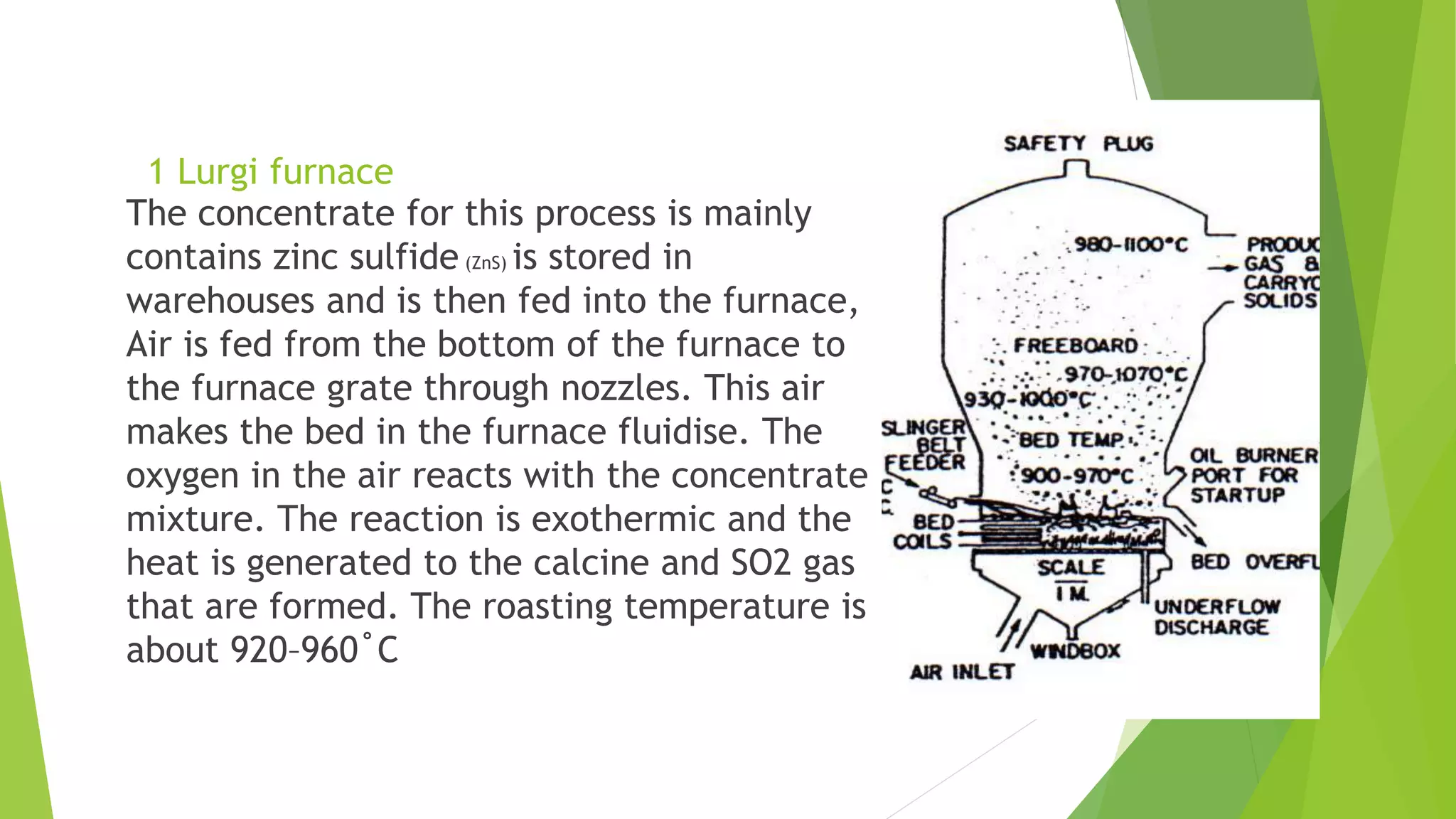
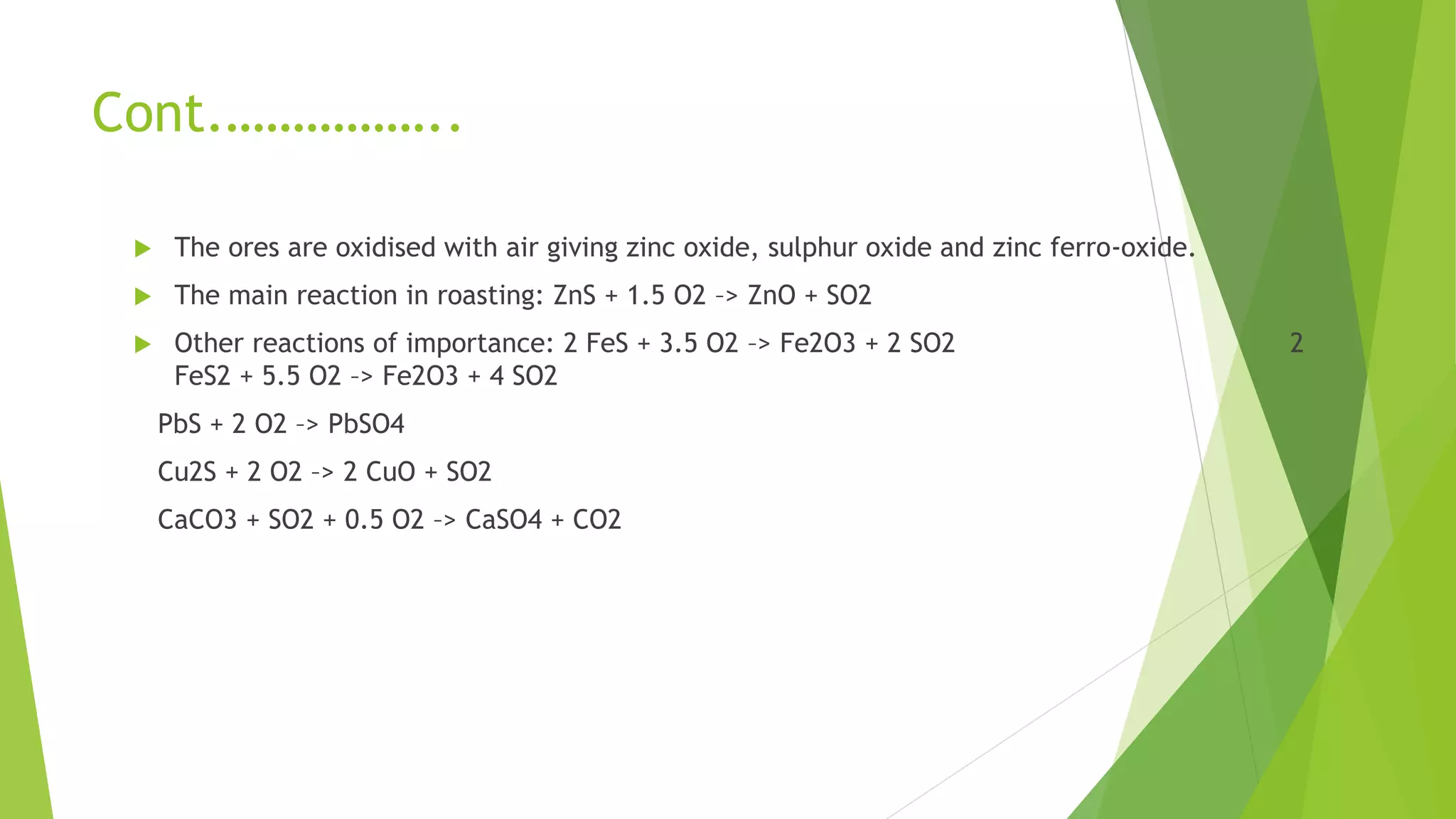
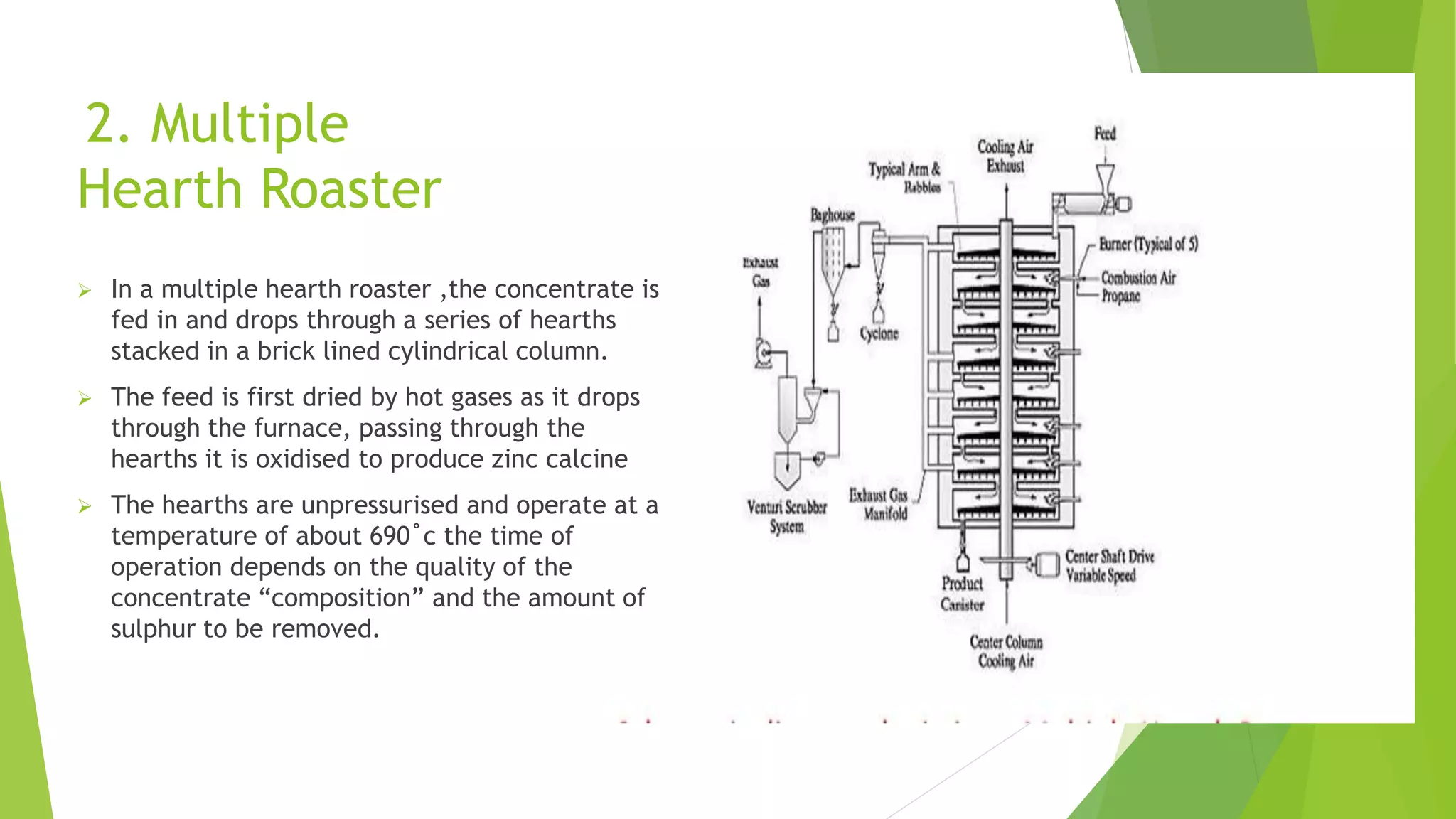
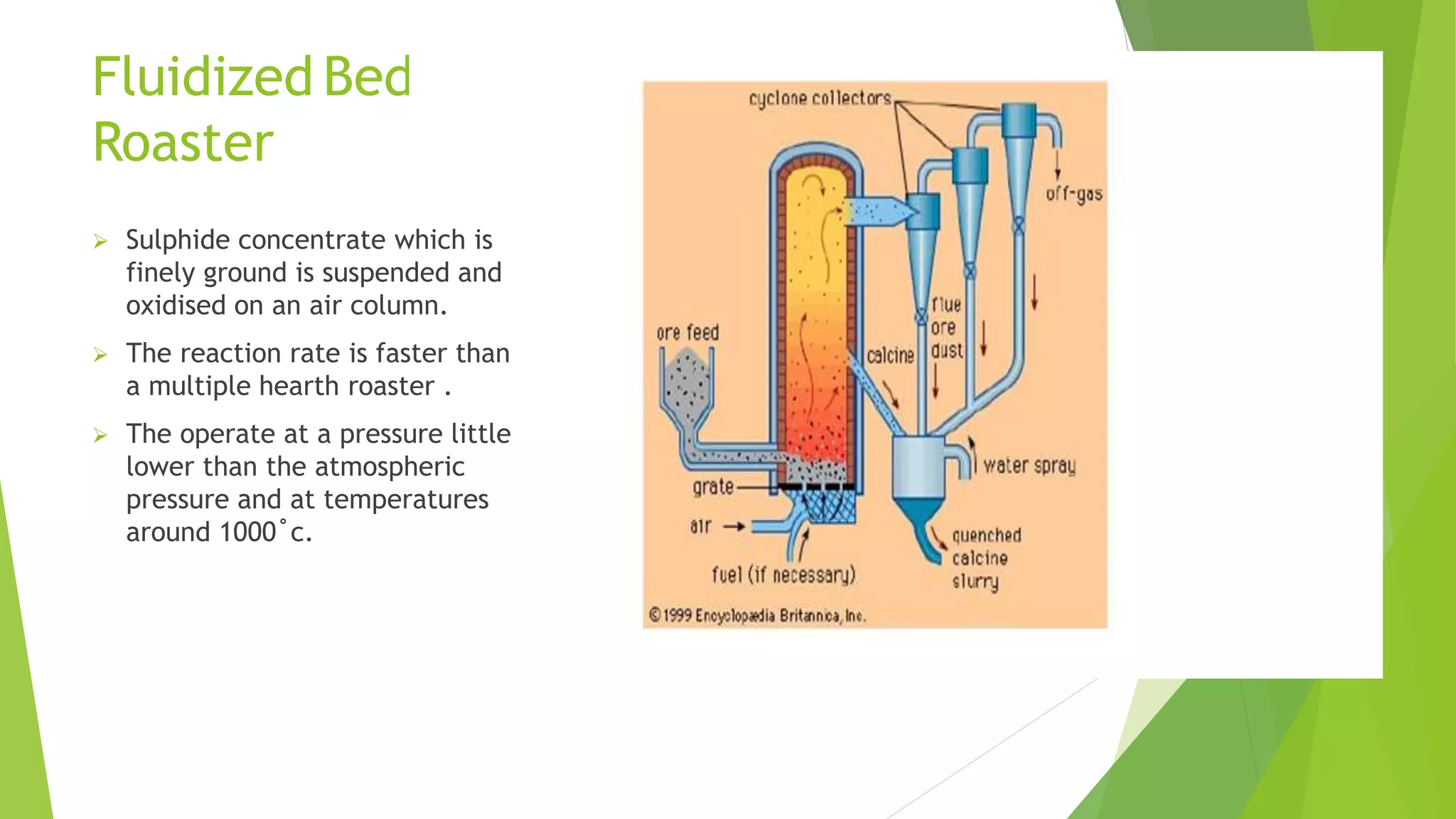
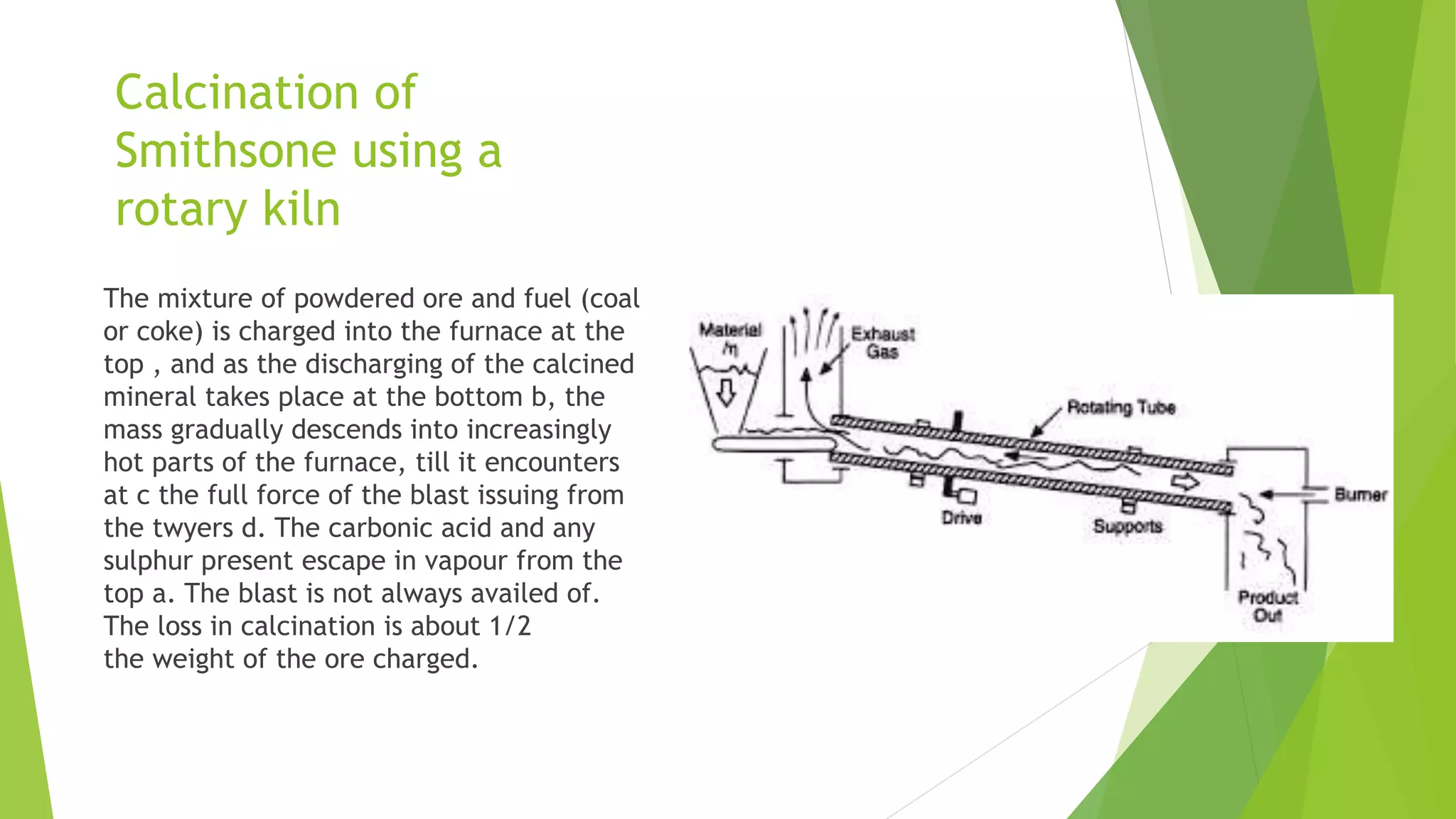
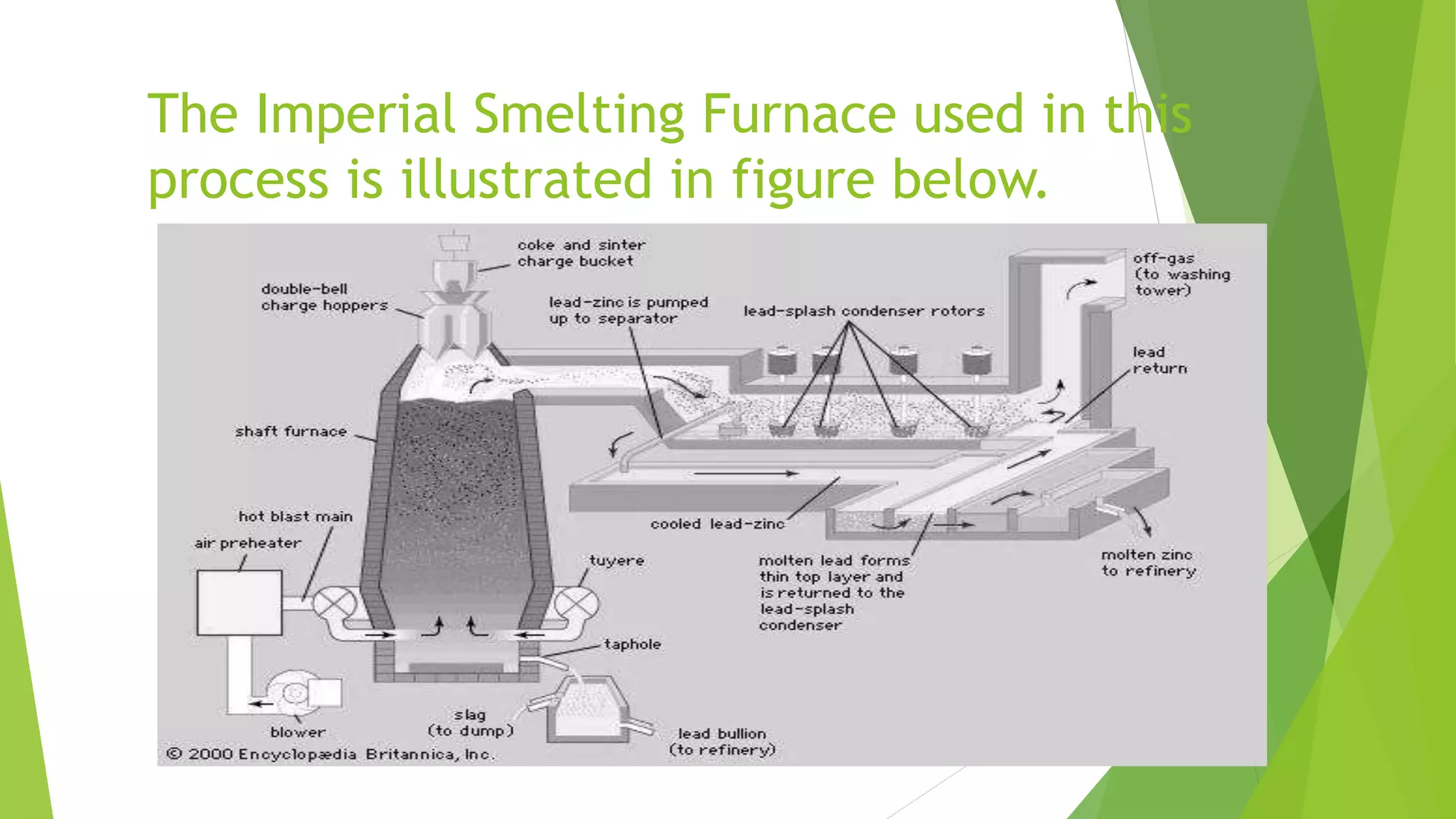
The document details the extraction of zinc through pyrometallurgical processes, highlighting its properties, common ores, and the methods of extraction including roasting and smelting. Key zinc minerals like sphalerite and smithsonite are discussed, along with the major global zinc reserves found primarily in North America and Australia. The document also explains the different roasting techniques and the process for producing zinc from ores, which includes steps like calcination and thermal smelting.



![Zinc ores
Zinc ores are widely distributed throughout the world, although more than 40
percent of the world’s output originates in North America and Australia. The
common zinc-containing minerals are the zinc sulfide known as zinc blende or
sphalerite (ZnS), a ferrous form of zinc blende known as marmatite [(ZnFe)S],
and a zinc carbonate known as calamine or smithsonite (ZnCO3).
The geology of zinc deposits is complex. In most cases, hydrothermal
mechanisms have occurred in which aqueous solutions were forced through
porous strata at high temperatures and pressures to dissolve zinc,](https://image.slidesharecdn.com/extractionofzincpresentation-181121113946/75/Pyrometallurgy-Extraction-of-zinc-presentation-4-2048.jpg)