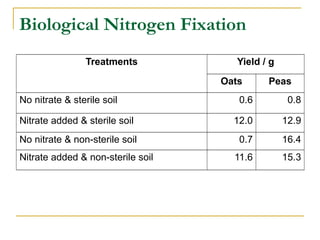
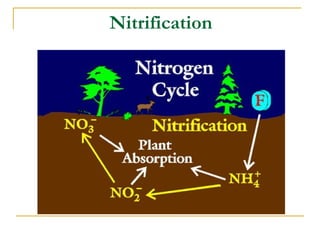
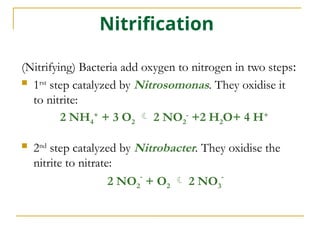
The document describes the nitrogen cycle and its significance in ecosystems, highlighting processes such as nitrogen fixation, ammonification, nitrification, and denitrification. It outlines the roles of nitrogen-fixing bacteria and human impacts on nitrogen fixation, including the use of synthetic fertilizers and fossil fuel consumption. Additionally, the document addresses environmental concerns such as eutrophication and pollution caused by nitrogen compounds.