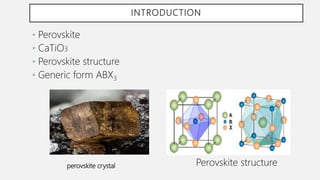
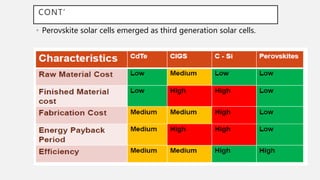
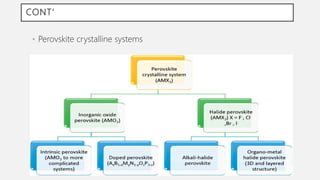
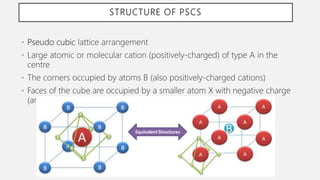
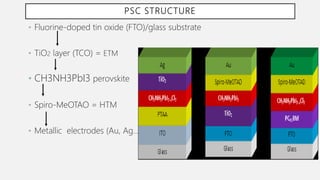
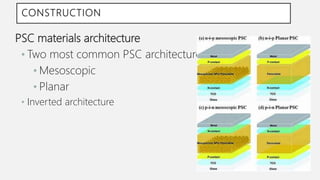
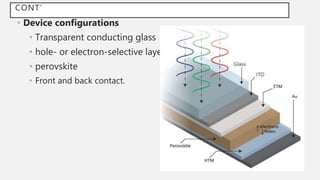
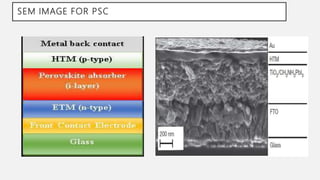
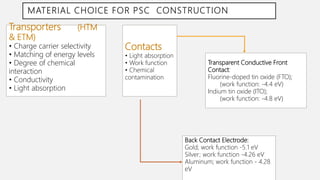
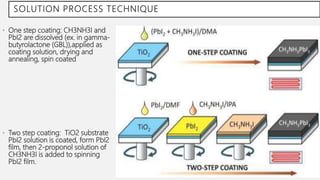
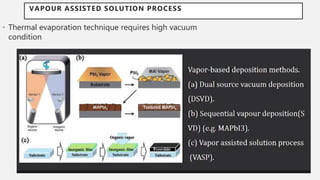
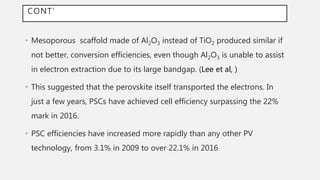
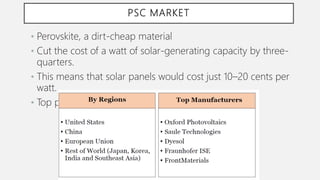
Perovskite solar cells (PSCs) are a promising third generation solar cell technology that can be produced at low cost with high efficiencies. PSCs use a perovskite crystalline structure as the light absorber layer, such as methylammonium lead iodide. Since their introduction in 2009 with an efficiency of 3.8%, PSC efficiencies have rapidly increased to over 22% due to improved materials and device architectures. While still facing challenges such as stability, PSCs have the potential to dramatically reduce the cost of solar electricity if further developed and commercialized.