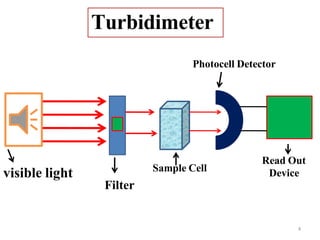
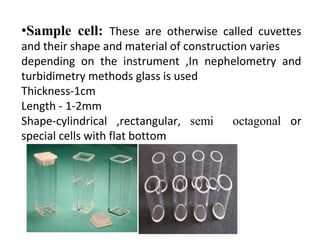
Nephelometry and turbidimetry are techniques that measure light scattering by particles in solution. Turbidimetry measures the intensity of transmitted light, while nephelometry measures the intensity of scattered light. Both techniques rely on the principle that as particle concentration increases, more light is scattered and less is transmitted. The amount of scattering depends on factors like particle size, concentration, and wavelength of light. These techniques can be used to analyze substances in water, biochemical samples, and other solutions through measurement of scattered or transmitted light.